 |
 |
- Search
| Intest Res > Volume 20(2); 2022 > Article |
|
Abstract
Background/Aims
Methods
Results
Conclusions
Supplementary Material
ADDITIONAL INFORMATION
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
This was an investigator-initiated study. There was no involvement of the drug manufacturer in either the conception, data collection, analysis, or manuscript writing. Rubin DT is a consultant to AbbVie, AbGenomics, Allergan Inc., Amgen, Celgene Corporation, Forward Pharma, Genentech/Roche, Janssen Pharmaceuticals, Merck & Co Inc., Miraca Life Sciences, Mitsubishi Tanabe Pharma Development America Inc., Napo Pharmaceuticals, Shire, Takeda, and Target Pharma Solutions and has received grant support from AbbVie, Genentech/Roche, Janssen Pharmaceuticals, Prometheus Laboratories, Shire, Takeda, and UCB Pharma. Cohen RD serves on the speaker’s bureau for AbbVie and Takeda; serves as a consultant for AbbVie, Celgene, Janssen, Pfizer, and Takeda; and has conducted clinical trials for AbbVie, Boehringer Ingelheim, Celgene Corp., Crohn’s & Colitis Foundation of America, Genentech, Gilead, Hollister, Medimmune, Pfizer, Receptos, Schwarz Pharma, Seres Therapeutics, and Takeda. His spouse serves on the Board of Directors for Aerpio Therapeutics, Novus Therapeutics, and Vital Therapies, Inc. Pekow J is a consultant for Versatem and CVS Caremark and has received grant support from AbbVie and Takeda. He has served on the advisory board for Takeda, Pfizer and Jansen. Dalal SR is on the speaker’s bureau of AbbVie and has served as a consultant for Pfizer. The other authors declare no conflict of interest related to this work.
Rubin DT is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author Contribution
Guarantor of article: Ayoub F. Conceptualization: Ayoub F, Odenwald M, Micic D, Dalal SR, Pekow J, Cohen RD, Rubin DT, Sakuraba A. Data curation: Ayoub F, Odenwald M. Formal analysis: Ayoub F. Investigation: Odenwald M, Dalal SR, Pekow J, Cohen RD, Rubin DT, Sakuraba A. Methodology: Ayoub F, Odenwald M. Project administration: Ayoub F. Software: Ayoub F. Supervision: Micic D, Dalal SR, Pekow J, Cohen RD, Rubin DT, Sakuraba A. Writing - original draft: Ayoub F. Writing - review & editing: Ayoub F, Odenwald M, Micic D. Approval of final manuscript: all authors.
Fig. 1.
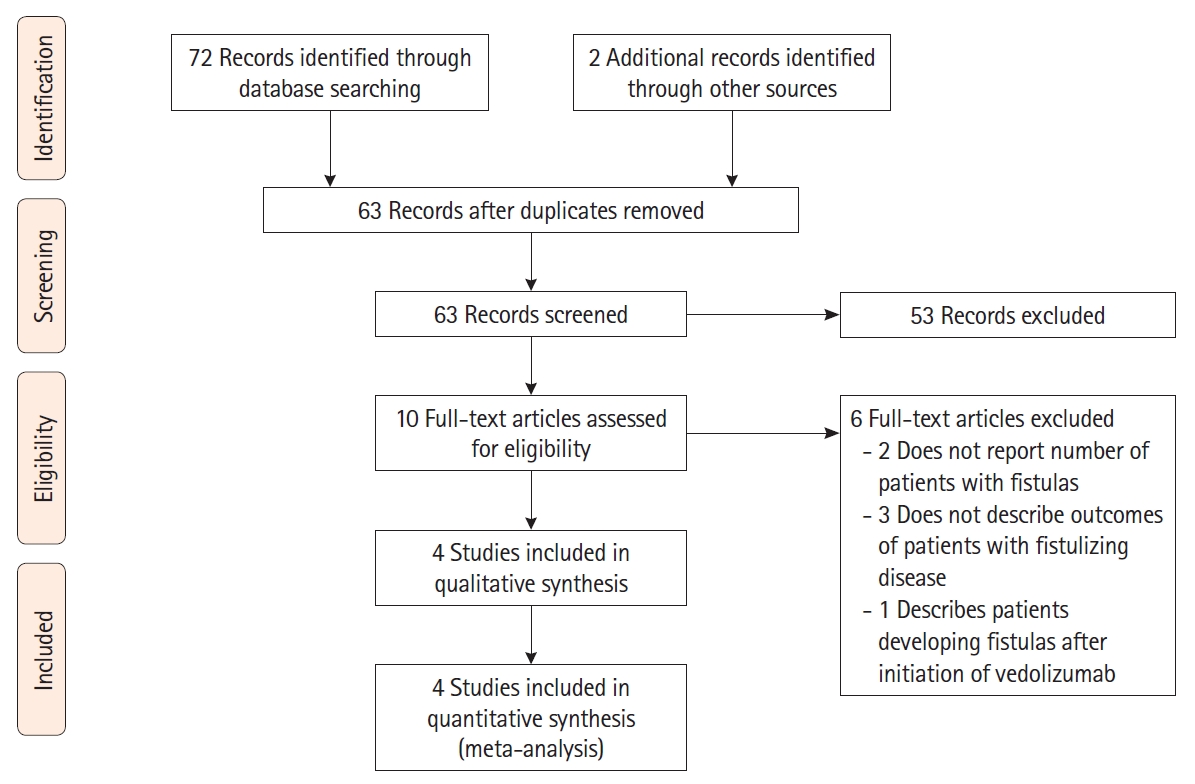
Fig. 2.
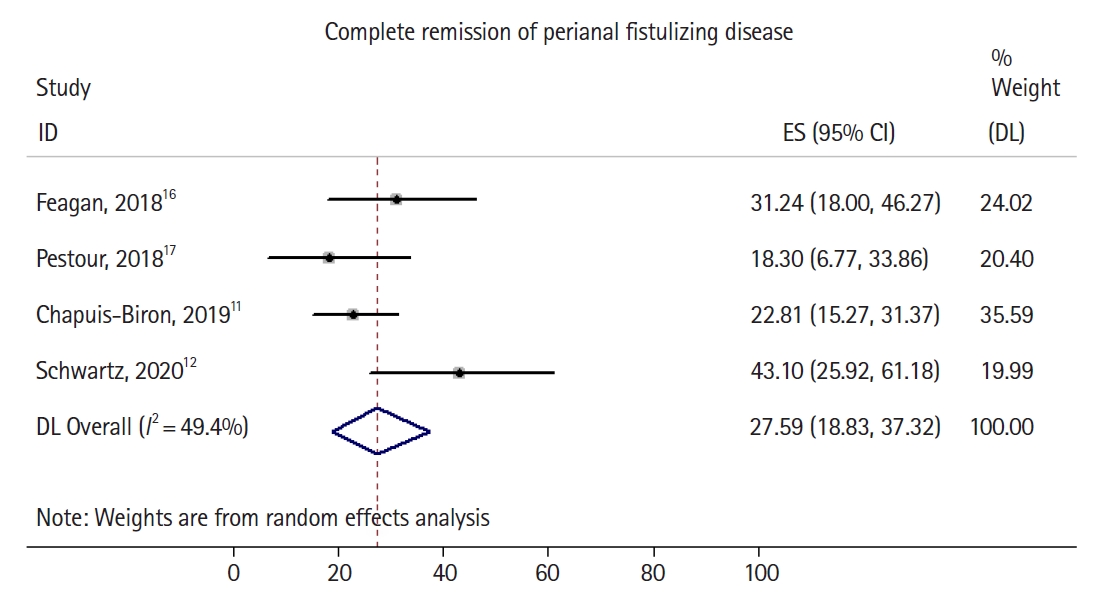
Fig. 3.
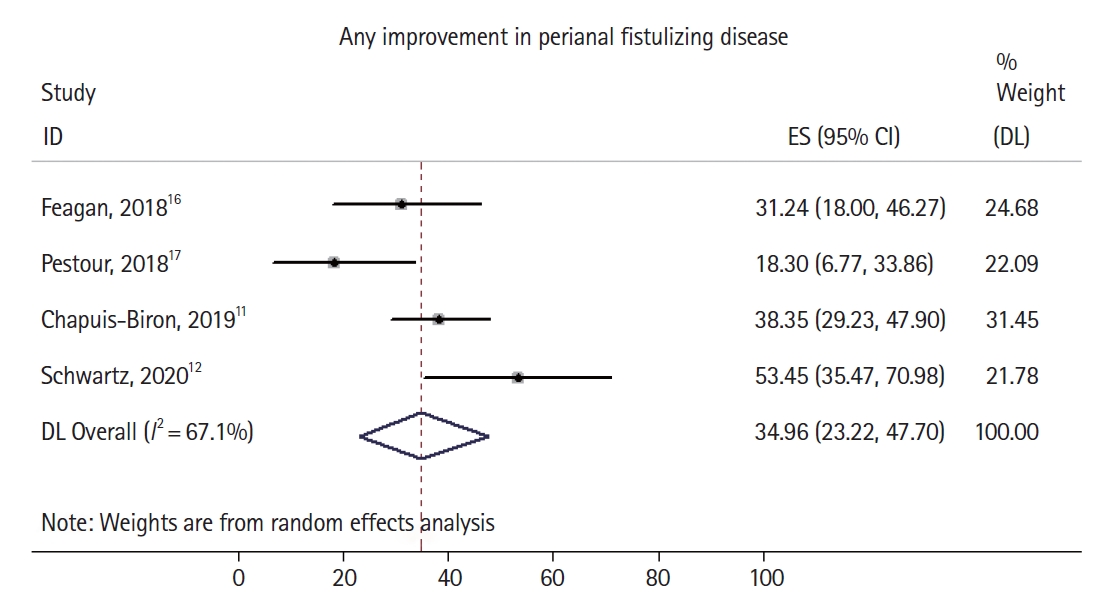
Table 1.
| First author (year) | Country | Type of study | Patients with active perianal disease | Age (yr) | Male sex | Modality for assessment of fistula healing | Final time point for assessment for fistula healing (wk) | Previous therapies | Patients with setons at time of induction with VDZ | Patients on concomitant immunomodulator therapy | Patients on concomitant antibiotic therapy | Patients on concomitant steroid therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pestour (2018) [17] | France | Multicenter retrospective cohort | 29 | NR | NR | NR | 24 | All patients had failed prior anti-TNF therapy | NR | NR | NR | NR |
| Feagan (2018) [16] | International | Multicenter randomized, placebo-controlled trial | 39 (18 in placebo maintenance arm) | 32.6 ± 9.5 | 20 | Clinical examination | 52 | 19 (49%) patients had failed prior anti-TNF therapy | NR | 14 | 21 | 23 |
| Chapuis-Biron (2019) [11] | France | Multicenter retrospective cohort | 102 | 38.9 ± 10.8 | 33 | Clinical examination +/- imaging | 24 | All patients had failed prior anti-TNF therapy | 61 | 45 | 39 | NR |
| Schwartz (2020) [12] | International | Multicenter randomized, controlled trial of 2 VDZ dosing regimens | 28 | 35.1 ± 10.4 | 21 | Clinical examination + imaging | 30 | 22 (79%) patients had failed prior anti-TNF therapy | NR | 7 | NR | 5 |
Table 2.
| First author (year) | Treatment arm | Patients with active perianal disease | Patients with complete healing of fistulizing disease | Patients at least partial healing of fistulizing disease | Dose escalation regimen | Patients requiring dose escalation | Additional therapy (antibiotics or surgery) |
|---|---|---|---|---|---|---|---|
| Pestour (2018) [17] | Standard dose VDZ induction and maintenancea | 29 | 5 (17) | NR (assumed to be same 5 patients with complete healing) | If no clinical response at week 6, additional infusion of 300 mg VDZ at week 10, and then once every 4 weeks | NR | NR |
| Feagan (2018) [16] | Standard dose VDZ induction, maintenance with dosing either every 4 weeks or every 8 weeks, both groups combineda | 39 | 12 (31) | NR (assumed to be same 12 patients with | None | None | Medical treatment (antibiotics) for 21/39 |
| VDZ induction, followed by placebo maintenance | 18 | 2 (11) | NR (assumed to be same 2 patients with complete healing) | ||||
| Chapuis-Biron (2019) [11] | Standard dose VDZ induction and maintenancea | 102 | 23 (23) | 39 (38) | Patients requiring escalation had 300 mg intravenously every 4 or 6 weeks or 600 mg every 8 weeks | 62 | Medical treatment (antibiotics) for 39/102 (38%) patients and surgical treatment for 41/102 (40%) patients |
| Schwartz (2020) [12] | VDZ 300 mg IV at weeks (0, 2, 6, 14, and 22) | 14 | 7 (50) | 9 (64.3) | None | None | NR |
| The same regimen plus an additional VDZ dose at week 10 | 14 | 5 (35.7) | 6 (42.9) |








