 |
 |
- Search
| Intest Res > Volume 21(2); 2023 > Article |
|
Abstract
Background/Aims
Methods
Results
ADDITIONAL INFORMATION
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
Sood A is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author Contribution
Conceptualization: Sood A. Data curation: Singh A, Narang V, Singh D, Barnaba Durairaj MV, Dutta AK, Sood A. Formal analysis: Singh A, Narang V, Bansal N, Sood A. Investigation: Singh A. Methodology: Gupta YK, Singh A, Sood A. Project administration: Midha V, Dutta AK, Sood A. Resources: Gupta YK, Singh A, Midha V, Mahajan R, Mehta V, Barnaba Durairaj MV, Dutta AK, Sood A. Supervision: Midha V, Dutta AK, Sood A. Visualization: Singh A, Dutta AK, Sood A. Writing - original draft: Singh A, Narang V. Writing - review & editing: Gupta YK, Singh A, Narang V, Mahajan R, Mehta V, Singh D, Bansal N, Barnaba Durairaj MV, Dutta AK, Sood A. Approval of final manuscript: all authors.
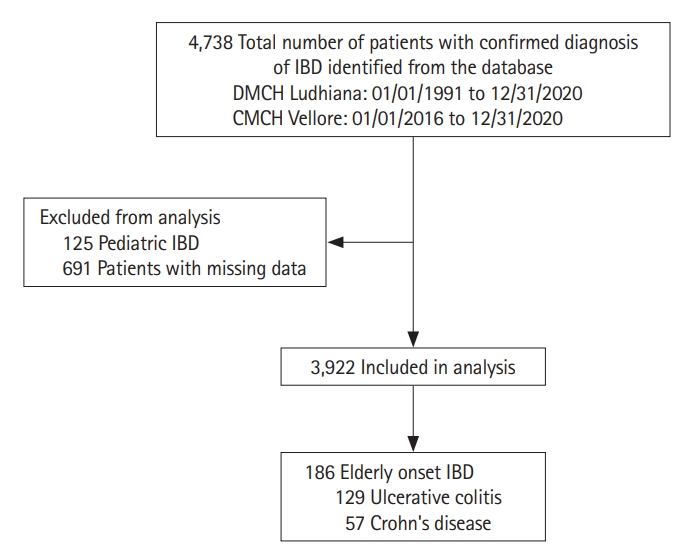
Fig.┬Ā1.
Table┬Ā1.
Table┬Ā2.
| Variable | Elderly onset CD (n=57) | Elderly onset UC (n=129) | P-value |
|---|---|---|---|
| Age at diagnosis (yr) | 68.18 ┬▒ 15.70 | 65.88 ┬▒ 7.58 | 0.170 |
| Duration of symptoms (yr) | 1.42 ┬▒ 5.62 | 1.88 ┬▒ 2.54 | 0.440 |
| Male sex | 36 (63.15) | 80 (62.01) | 0.880 |
| Addictions | |||
| ŌĆāSmoking | 8 (14.03) | 10 (7.75) | 0.180 |
| ŌĆāAlcohol | 6 (10.52) | 13 (10.07) | 0.920 |
| ŌĆāOpium | - | 3 (2.32) | 0.240 |
| Comorbid illnessesa | 22 (38.59) | 50 (38.75) | 0.980 |
| Family history of IBD | - | 2 (1.55) | 0.340 |
| Clinical features | |||
| ŌĆāAbdominal pain | 48 (84.21) | 85 (65.89) | 0.010 |
| ŌĆāFever | 11 (19.29) | 26 (20.15) | 0.890 |
| ŌĆāIncreased frequency of stools | 31 (54.38) | 119 (92.24) | < 0.001 |
| ŌĆāNocturnal frequency of stools | 2 (3.50) | 86 (66.66) | < 0.001 |
| ŌĆāBlood in stools | 5 (8.77) | 104 (80.62) | < 0.001 |
| ŌĆāLoss of appetite | 24 (42.10) | 39 (30.23) | 0.110 |
| ŌĆāWeight loss | 35 (61.40) | 65 (50.38) | 0.160 |
| ŌĆāNausea & vomiting | 28 (49.12) | 4 (3.10) | < 0.001 |
| Extraintestinal manifestations | |||
| ŌĆāMusculoskeletal | 15 (26.31) | 41 (31.78) | 0.450 |
| ŌĆāOcular | 1 (1.75) | 1 (0.77) | 0.550 |
| ŌĆāDermatological | - | - | - |
| ŌĆāPrimary sclerosing cholangitis | - | 1 (0.77) | 0.500 |
| ŌĆāNephrolithiasis | 1 (1.75) | - | 0.130 |
| ŌĆāThrombotic complications | - | - | - |
| ŌĆāOral ulcers | 5 (8.77) | 10 (7.75) | 0.770 |
| Disease severity at diagnosis | |||
| ŌĆāMild | 18 (31.57) | 32 (24.80) | 0.330 |
| ŌĆāModerate | 37 (64.91) | 90 (69.76) | 0.510 |
| ŌĆāSevere | 2 (3.50) | 7 (5.42) | 0.570 |
| Disease extent at diagnosis | - | - | |
| ŌĆāProctitis | 22 (17.05) | ||
| ŌĆāLeft-sided colitis | 77 (59.68) | ||
| ŌĆāPancolitis | 30 (23.25) | ||
| Disease location | - | - | |
| ŌĆāL1 | 26 (45.61) | ||
| ŌĆāL2 | 17 (29.82) | ||
| ŌĆāL3 | 13 (22.80) | ||
| ŌĆāL1+L4 | 1 (1.75) | ||
| Disease behavior | - | - | |
| ŌĆāB1 | 36 (63.15) | ||
| ŌĆāB2 | 17 (29.82) | ||
| ŌĆāB3 | 4 (7.01) | ||
| Perianal disease | 3 (5.26) | - | - |
| Treatment ever received | |||
| ŌĆā5-Aminosalicylates | 47 (82.45) | 129 (100) | < 0.001 |
| ŌĆāThiopurines | 6 (10.52) | 13 (10.08) | 0.920 |
| ŌĆāBiologics | - | 4 (3.10) | 0.180 |
| ŌĆāCorticosteroids | 39 (68.42) | 52 (40.31) | < 0.001 |
| ŌĆāSurgery | 5 (8.77) | 8 (6.20) | 0.520 |
| ŌĆāŌĆāFailure of medical therapy | - | 1 (0.77) | 0.500 |
| ŌĆāŌĆāColorectal cancer | 1 (1.75) | 7 (5.42) | 0.250 |
| ŌĆāŌĆāIntestinal perforation | - | - | - |
| ŌĆāŌĆāToxic megacolon | - | - | - |
| ŌĆāŌĆāIntestinal obstruction | 4 (7.01) | - | 0.002 |
Table┬Ā3.
| Variable |
UC |
CD |
||||||
|---|---|---|---|---|---|---|---|---|
| Elderly onset UC (n=129) | Adult onset UC (n=3,043) | P-value | Elderly onset CD (n=57) | Adult onset CD (n=693) | P-value | |||
| Male sex | 80 (62.01) | 1,661 (54.58) | 0.100 | 36 (63.15) | 398 (57.43) | 0.400 | ||
| Age at diagnosis (yr) | 65.88 ┬▒ 7.58 | 34.33 ┬▒ 10.32 | < 0.001 | 68.18 ┬▒ 15.70 | 36.70 ┬▒ 15.50 | < 0.001 | ||
| Duration of symptoms before diagnosis (yr) | 1.88┬▒2.54 | 3.44 ┬▒ 4.91 | < 0.001 | 1.42 ┬▒ 5.62 | 2.99 ┬▒ 4.54 | 0.010 | ||
| Addictions | ||||||||
| ŌĆā | Smoking | 10 (7.75) | 76 (2.49) | < 0.001 | 8 (14.03) | 38 (5.48) | 0.009 | |
| Alcohol | 13 (10.07) | 224 (7.36) | 0.250 | 6 (10.52) | 120 (17.31) | 0.180 | ||
| Opium | 3 (2.32) | 24 (0.78) | 0.060 | - | 20 (2.88) | 0.190 | ||
| Co-morbid illnesses | ||||||||
| Coronary artery disease | 7 (5.42) | 11 (0.36) | < 0.001 | 3 (5.26) | 1 (0.14) | < 0.001 | ||
| Diabetes mellitus | 27 (20.93) | 94 (3.08) | < 0.001 | 5 (8.77) | 19 (2.74) | 0.010 | ||
| Hypertension | 16 (12.40) | 48 (1.57) | < 0.001 | 5 (8.77) | 10 (1.44) | < 0.001 | ||
| Othersa | 4 (3.10) | 10 (0.32) | 0.520 | 5 (8.77) | 30 (4.32) | 0.120 | ||
| Family history of IBD | 2 (1.55) | 116 (3.81) | 0.180 | - | 9 (1.29) | 0.390 | ||
| Previous appendectomy | - | 27 (0.88) | 0.280 | - | 9 (1.29) | 0.390 | ||
| Clinical features | ||||||||
| Abdominal pain | 85 (65.89) | 1,836 (60.33) | 0.200 | 48 (84.21) | 532 (76.76) | 0.190 | ||
| Fever | 26 (20.15) | 587 (19.29) | 0.800 | 11 (19.29) | 237 (34.19) | 0.020 | ||
| Increased frequency of stools | 119 (92.24) | 2,469 (81.13) | 0.001 | 31 (54.38) | 401 (57.86) | 0.600 | ||
| Nocturnal frequency of stools | 86 (66.66) | 1,775 (58.33) | 0.060 | 2 (3.50) | 34 (4.90) | 0.630 | ||
| Blood in stools | 104 (80.62) | 2,324 (76.37) | 0.260 | 5 (8.77) | 215 (31.02) | < 0.001 | ||
| Loss of appetite | 39 (30.23) | 1,316 (43.24) | 0.003 | 24 (42.10) | 338 (48.77) | 0.330 | ||
| Weight loss | 65 (50.38) | 774 (25.43) | < 0.001 | 35 (61.40) | 444 (64.06) | 0.680 | ||
| Nausea & vomiting | 4 (3.10) | 100 (3.28) | 0.910 | 28 (49.12) | 304 (43.86) | 0.440 | ||
| Mayo Clinic score at diagnosis | 6.98 ┬▒ 2.04 | 7.25 ┬▒ 2.35 | 0.190 | - | - | - | ||
| CDAI at diagnosis | - | - | - | 231.14 ┬▒ 29.69 | 258.45 ┬▒ 31.20 | < 0.001 | ||
| Disease severity | ||||||||
| Mild | 32 (24.80) | 595 (19.55) | 0.140 | 18 (31.57) | 153 (22.07) | 0.10 | ||
| Moderate | 90 (69.76) | 2,037 (66.94) | 0.500 | 37 (64.91) | 480 (69.26) | 0.49 | ||
| Severe | 7 (5.42) | 411 (13.50) | 0.007 | 2 (3.50) | 60 (8.65) | 0.17 | ||
| Disease extent | - | - | - | |||||
| Proctitis | 22 (17.05) | 579 (19.03) | 0.570 | |||||
| Left-sided colitis | 77 (59.68) | 1,726 (56.72) | 0.500 | |||||
| Pancolitis | 30 (23.25) | 738 (24.25) | 0.790 | |||||
| Disease location | - | - | - | |||||
| L1 | 26 (45.61) | 314 (45.31) | 0.960 | |||||
| L2 | 17 (29.82) | 203 (29.29) | 0.930 | |||||
| L3 | 13 (22.80) | 160 (23.08) | 0.960 | |||||
| L1+L4 | 1 (1.75) | 16 (2.30) | 0.780 | |||||
| Disease behavior | - | - | - | |||||
| B1 | 36 (63.15) | 502 (72.43) | 0.130 | |||||
| B2 | 17 (29.82) | 148 (21.35) | 0.130 | |||||
| B3 | 4 (7.01) | 43 (6.20) | 0.800 | |||||
| Perianal disease | - | - | - | 3 (5.26) | 28 (4.04) | 0.650 | ||
| Extraintestinal manifestations | ||||||||
| Musculoskeletal | 41 (31.78) | 401 (13.17) | < 0.001 | 15 (26.31) | 129 (18.61) | 0.150 | ||
| Ocular | 1 (0.77) | 16 (0.52) | 0.700 | 1 (1.75) | 8 (1.15) | 0.680 | ||
| Dermatological | - | 20 (0.65) | 0.350 | - | 5 (0.72) | 0.520 | ||
| Primary sclerosing cholangitis | 1 (0.77) | 9 (0.29) | 0.330 | - | 3 (0.43) | 0.620 | ||
| Nephrolithiasis | - | 5 (0.16) | 0.640 | 1 (1.75) | 7 (1.01) | 0.600 | ||
| Thrombotic complications | - | 5 (0.16) | 0.640 | - | 1 (0.14) | 0.770 | ||
| Oral ulcers | 10 (7.75) | 295 (9.69) | 0.460 | 5 (8.77) | 53 (7.64) | 0.750 | ||
| Treatment | ||||||||
| 5-Aminosalicylates | 129 (100) | 3,031 (99.60) | 0.470 | 47 (82.45) | 560 (80.80) | 0.760 | ||
| Thiopurines | 13 (10.08) | 1,217 (39.99) | < 0.001 | 6 (10.52) | 500 (72.15) | < 0.001 | ||
| Biologics | 4 (3.10) | 196 (6.44) | 0.120 | - | 32 (4.61) | 0.100 | ||
| Corticosteroids | 52 (40.31) | 1,462 (48.04) | 0.080 | 39 (68.42) | 465 (67.09) | 0.830 | ||
| Surgery | 8 (6.20) | 114 (3.74) | 0.190 | 5 (8.77) | 52 (7.50) | 0.720 | ||
| ŌĆā | Failure of medical therapy | 1 (0.77) | 80 (2.62) | 0.190 | - | 3 (0.43) | 0.620 | |
| Colorectal cancer | 7 (5.42) | 21 (0.69) | < 0.001 | 1 (1.75) | 2 (0.29) | 0.090 | ||
| Intestinal perforation | - | 3 (0.09) | 0.730 | - | 8 (1.15) | 0.410 | ||
| Toxic megacolon | - | 10 (0.32) | 0.520 | - | - | - | ||
| Intestinal obstruction | - | - | - | 4 (7.01) | 36 (5.19) | 0.550 | ||
| Perianal disease | - | - | - | - | 3 (0.43) | 0.620 | ||
IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, CrohnŌĆÖs disease; CDAI, CrohnŌĆÖs Disease Activity Index; L1, ileal disease; L2, colonic disease; L3, ileo-colonic disease; L4, upper gastrointestinal disease; B1, non-penetrating, non-stricturing disease; B2, stricturing disease; B3, penetrating disease.








