Concomitant ankylosing spondylitis can increase the risk of biologics or small molecule therapies to control inflammatory bowel disease
Article information
Abstract
Background/Aims
Patients with inflammatory bowel disease (IBD) are diagnosed with ankylosing spondylitis (AS) often. However, the disease course of patients with both IBD and AS is not well understood. This study aims to evaluate the effect of concomitant AS on IBD outcomes.
Methods
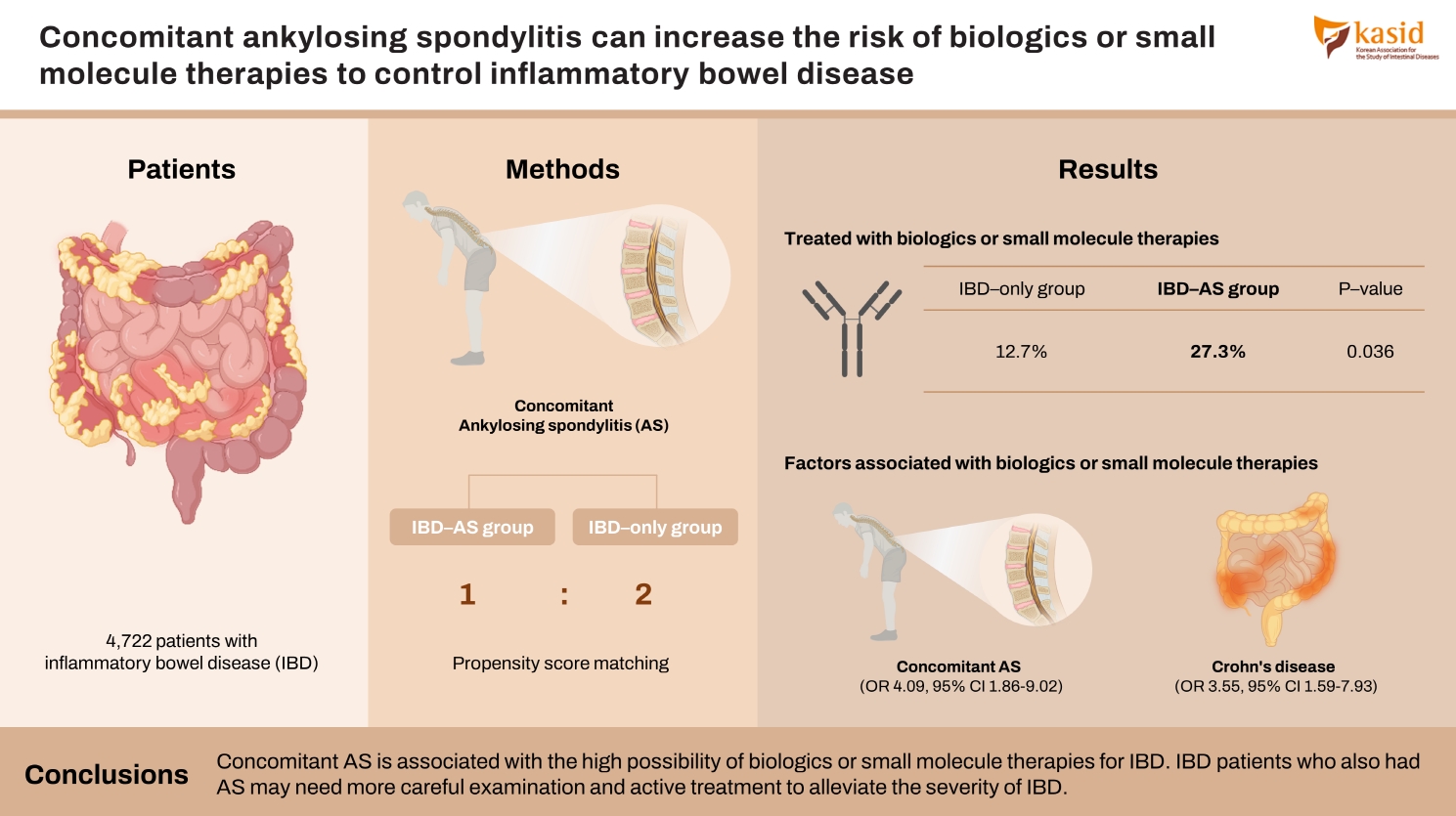
Among the 4,722 patients with IBD who were treated in 3 academic hospitals from 2004 to 2021, 55 were also diagnosed with AS (IBD-AS group). Based on patients’ electronic medical records, the outcomes of IBD in IBD-AS group and IBD group without AS (IBD-only group) were appraised.
Results
The proportion of patients treated with biologics or small molecule therapies was significantly higher in IBD-AS group than the proportion in IBD-only group (27.3% vs. 12.7%, P=0.036). Patients with both ulcerative colitis and AS had a significantly higher risk of biologics or small molecule therapies than patients with only ulcerative colitis (P<0.001). For univariable logistic regression, biologics or small molecule therapies were associated with concomitant AS (odds ratio, 4.099; 95% confidence interval, 1.863–9.021; P<0.001) and Crohn’s disease (odds ratio, 3.552; 95% confidence interval, 1.590–7.934; P=0.002).
Conclusions
Concomitant AS is associated with the high possibility of biologics or small molecule therapies for IBD. IBD patients who also had AS may need more careful examination and active treatment to alleviate the severity of IBD.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of gastrointestinal tract and is classified as ulcerative colitis (UC) and Crohn’s disease (CD). The pathogenesis of IBD is not well established but multiple factors, including genetic, immunologic, microbial, and environmental factors, might comprehensively develop and aggravate IBD [1]. Patients with moderate-to-severe IBD are required immunomodulators (IMMs), biologics, or small molecule therapies and have high risks of hospitalization or surgery. It is important to predict the outcome of IBD, determine its treatment, and reassess the strategy according to the expected outcome.
Arthritis is one of the extragastrointestinal manifestations that can be observed in patients with IBD. Arthritis in IBD patients was divided into 2 different clinical patterns: peripheral arthritis and axial arthritis. Major clinical characteristics of ankylosing spondylitis (AS) are sacroiliitis, inflammation in the axial skeleton, peripheral arthritis, enthesitis, and anterior uveitis [2]. Long-term spinal inflammation of AS patients provokes low back pain, spinal stiffness, and restricted spinal mobility. About 6% to 14% of AS patients have IBD and the prevalence and incidence of IBD in AS patients were higher than in those general population [3]. Moreover, concomitant IBD in AS patients was associated with high spinal pain scores and poor physical function [4]. Bone marrow edema and erosion scored using Spondyloarthritis Research Consortium of Canada (SPARCC) method in AS patients had a positive correlation with the severity of histologically chronic enterocolitis [5].
On the other hand, a considerable number of IBD patients have clinical manifestations related to AS. The prevalence of AS in patients with IBD was in a range of 4%–10%, which was higher than that in the general population (0.32%–0.07%) [6-8]. The correlation of concomitant AS and IBD outcome is still not clear due to the lack of relevant studies. Therefore, we conducted a retrospective cohort study to investigate whether concomitant AS is related to IBD outcomes.
METHODS
1. Patients
The data was extracted from the electronic medical records of 3 hospitals; Seoul National University Hospital (SNUH), Seoul National University Bundang Hospital (SNUBH), and Seoul Metropolitan Government Seoul National University (SMG-SNU) Boramae Medical Center. A retrospective cohort study included all patients who were diagnosed with both IBD and AS (IBD-AS group) in the 3 hospitals. Patients with AS were first identified by an International Classification of Disease, Tenth Revision (ICD-10) code of M45.0-45.9. The diagnosis of AS was confirmed according to the modified New York criteria by rheumatologic specialists [9]. We identified 55 patients in IBD-AS group and 4,665 patients in IBD-only group. The research was approved by the ethics committee of the 3 hospitals (IRB Nos: 2105-184-1222 in SNUH, B-2107-696-402 in SNUBH, 30-2019-134 in SMG-SNU Boramae Medical Center). The informed consent was waived.
2. Data Collection and Covariates
The initial data, which included outpatient and inpatient records, admission and discharge summaries, surgery records, nursing information, and examination results, were collected and organized. The following covariates were extracted: age, age at diagnosis for IBD or AS, sex, types of IBD, surgery, emergent room (ER) visit, biologics, small molecules, IMMs, and comorbidities.
Bowel resection was usually performed when patients failed their medical treatments or had medically intractable complications [10,11]. Due to the policies of Korean national health insurance, treatment with biologics, small molecules, or IMMs can reflect the severity of IBD. Top-down therapy, a treatment strategy that used high potent medications early before low potent medications, cannot be applied in the real-world clinical environment in Korea because of policies of national health insurance, where more than 98% of the Korean population is enrolled [12]. Because Korean national health insurance covered step-up therapy, biologics, small molecules, and IMMs cannot be used for patients with mild or early IBD, but only patients with moderate-to-severe IBD.
Surgery included any kinds of operations for IBD complications. Bowel resection included operations that removed all or part of the small intestine or colon. When an operation did not include procedures of bowel resection such as anal fistulectomy or sphincterotomy, it did not belong to bowel resection but to surgery. ER visit was defined as the cases in which patients visit ER because of the symptoms or complications of IBD. Biologics were defined as infliximab, adalimumab, golimumab, vedolizumab, and ustekinumab [13-19]. Administration of tofacitinib, a small-molecule kinase inhibitor, was also investigated. IMMs were defined as azathioprine, cyclosporine, mercaptopurine, methotrexate, leflunomide, and hydroxychloroquine. When we compared IBD outcomes of IBD-AS group with IBD-only group, medications prescribed for other diseases than IBD were excluded. We analyzed the following comorbidities: hypertension, type 2 diabetes mellitus, dyslipidemia, immune-mediated diseases, and gastrointestinal cancer [20].
3. Propensity Score Matching
Propensity score matching (PSM) was applied to match the IBD-AS group and IBD-only group due to the unequal distribution of demographics between 2 groups at their baselines. Age, sex, and type of IBD were used as the matching scores. The nearest neighbor method on the logit scale and the match of 1:2 ratio were used. After PSM, 55 patients were included in IBD-AS group and 110 patients in only IBD group. PSM matching was performed by R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
4. Statistical Analysis
Statistical analysis was done by R 4.2.1 (R Foundation for Statistical Computing) and IBM SPSS Statistics 25.0 (IBM Corp., Armonk, NY, USA). Patient characteristics and outcomes are summarized using standard summary statistics. Mean±standard deviation was used for continuous variables and number and percentage for categorical variables. Student t-test was calculated for continuous variables and Pearson chi-square test for categorical variables. We calculated the risk of a poor IBD outcome using univariable logistic regression, with variables, such as age, IBD–diagnosed age, sex, and concomitant AS. We considered a significance level of 0.05 as statistically significant.
RESULTS
1. Characteristics of IBD Patients with or without AS
Among the patients with IBD, 36 patients from SNUH, 13 from SNUBH, and 6 from SMG-SNU Boramae Medical Center were diagnosed with AS. IBD-AS group consisted of 14 CD patients (25.5%) and 41 UC patients (74.5%) (Table 1). Among patients with IBD-only group, 1,386 CD patients (29.8%) and 3,269 UC patients (70.2%) were not diagnosed with AS. IBD-AS patients were diagnosed with IBD at a significantly earlier age than IBD-only group. Thirty-five patients were diagnosed with IBD before AS (63.6%) and 17 patients were diagnosed with AS before IBD (30.9%).
The number of patients treated with biologics or small molecules was significantly larger in IBD-AS group than IBD-only group. In IBD-AS group, 15 (68.2%) and 7 (31.8%) patients were prescribed biologics or small molecules for the treatment of IBD and AS, respectively. Nearly two-thirds of IBD-AS and IBD-only patients experienced the treatment with only one type of biologics or small molecules. Adalimumab and infliximab were the most widely used biologics or small molecules in IBD-AS group and IBD-only group, respectively. There were no significant differences in the number and type of biologics between biologics or small molecules-treated IBD-AS group and IBD-only group.
According to the Montreal classification, more than half of CD-AS patients showed colon-involving disease extent (57.1%) and non-stricturing and non-penetrating behavior (57.1%). In UC patients, the most common disease extent was proctitis (41.2%) and the second most disease extent was left-side colitis (32.4%). The number of HLA-B27-positive patients was the same as in HLA-B27-negative patients in IBD-AS group. In IBD-AS group, 2 patients were diagnosed with pyoderma gangrenosum, 2 were diagnosed with uveitis, and 1 was diagnosed with non-axial arthritis. Most patients with IBD-AS (n = 44) took nonsteroidal anti-inflammatory drugs (NSAIDs) to relieve their axial arthralgia. Eight IBD-AS patients did not require the prescription of NSAIDs because their pain was tolerable without medication. The NSAIDs prescription information of 3 IBD-AS patients was not available.
2. Comparison of Clinical Characteristics According to Diagnosis Order in Patients with IBD and AS
Among IBD-AS patients, 37 patients were diagnosed with IBD before the onset of AS, and 15 patients were diagnosed with AS before the onset of IBD. There were no significant differences in age, age at diagnosis, sex, type of IBD, bowel resection, ER visit, treatment of biologics or small molecules, and C-reactive protein at diagnosis between patients who were diagnosed with IBD before the onset of AS and those who were diagnosed AS before the onset of IBD. However, the number of patients prescribed IMMs was significantly larger in patients who were diagnosed with AS before the onset of IBD than in those who were diagnosed with IBD before the onset of AS (Supplementary Table 1). Eleven and 4 IBD-AS patients were prescribed biologics for the treatment of IBD and AS, respectively. Only 2 IBD-AS patients were treated with IMMs for AS but 13 patients for IBD.
3. Effects of Concomitant AS on the Disease Course of IBD
After PSM, 55 patients of IBD-AS group and 110 patients of IBD-only group, whose age, sex, and IBD type matched, were identified (Fig. 1). The number of patients treated with biologics or small molecules was significantly higher in IBD-AS group than that of IBD-only group and IBD-AS group experienced more kinds of biologics or small molecules than IBD-only group (Table 2). Adalimumab and infliximab were the most widely used biologics or small molecules in IBD-AS group and IBD-only group, respectively. The period between the diagnosis of IBD and the initiation of the first biologics or small molecules was not significantly different between IBD-AS group (8.82 ± 2.29 years) and IBD-only group (6.57 ± 1.55 years) (P=0.489). There were no differences in the proportion of patients treated with IMMs but patients in IBD-AS group treated with IMM were more likely to change their first IMM to other ones than those in only IBD group. There were no significant differences in surgery, bowel resection, ER visit, and underlying diseases between both groups.
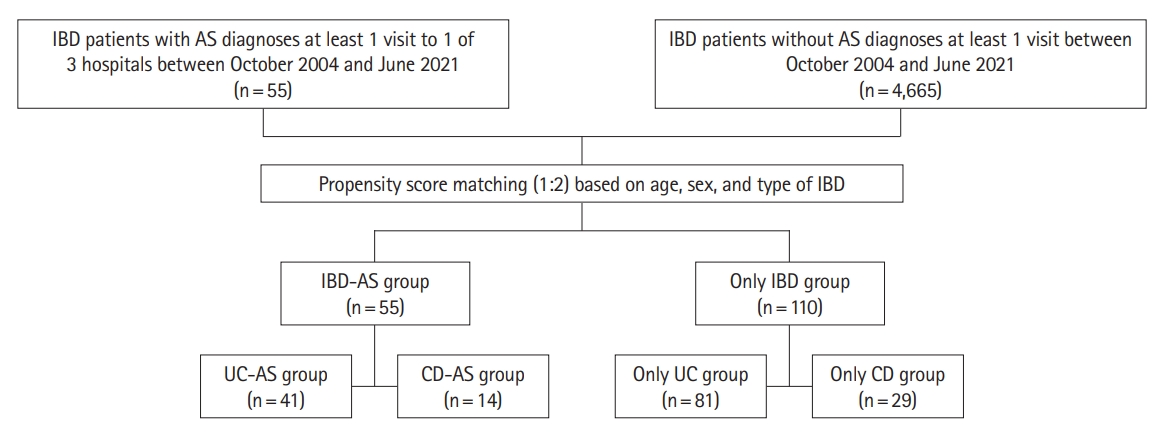
Flow diagram of patients selected and matched in IBD-AS group and IBD-only group. IBD, inflammatory bowel disease; AS, ankylosing spondylitis; UC, ulcerative colitis; CD, Crohn’s disease.
The characteristics of UC-AS group and matched only UC group was evaluated (Supplementary Table 2). The number of patients treated with biologics or small molecules was significantly greater in UC-AS group than in only UC group. UC-AS group had a higher risk of administration of more than one kind of biologics, small molecules, or IMMs. There were no significant differences in surgery, bowel resection, ER visit, and underlying diseases between UC-AS group and only UC group. The number of patients in CD-AS group and only CD group was 14 and 28, respectively. There were no significant differences in outcomes between only CD group and CD-AS group.
Univariable logistic regression analysis was performed to reveal risk factors for biologics or small molecule therapies (Table 3). Logistic regression analysis identified that concomitant AS and CD were significant risk factors for treatment with biologics or small molecules in patients with IBD.
DISCUSSION
Our present study revealed the characteristics and outcomes of IBD-AS patients, compared to patients with only IBD. IBD-AS patients had a higher risk of starting biologics or small molecules for IBD treatment than patients with only IBD. UC patients were likely to be treated with biologics or small molecules when they were also diagnosed with AS. There was no significant difference in IBD outcomes between CD-AS patients and patients with only CD.
Some studies showed similar results to our study. A Swiss cohort study reported that IBD patients with sacroiliitis or AS had more possibility to need anti-TNF agents or surgery for IBD treatment [21]. Not only anti-TNF agents but also corticosteroids and IMMs were more frequently treated for IBD patients with AS than those without AS [22]. However, the relationship between AS and the severity of CD was controversial [22,23].
Patients with IBD and AS have a high risk of severe IBD because of pathophysiological correlation between IBD and AS. First, IBD and AS have similar genetic polymorphisms. Single-nucleotide polymorphism research revealed that IL23R and kinase proteins related to IL23R signaling, STAT3, and JAK2 were associated with AS [24]. IL23R variants are related to inflammation in terminal ileum from CD patients and colon from UC patients. IL-23 subunit p19 is important for activation of IL-17-producing T cells in inflammatory reactions in IBD patients [25]. Moreover, in a study using the illumine Exomechip microarray, CDKAL1, C7orf72, and TLR10 genes were identified in both AS and IBD patients [26].
IBS and AS share similar immunologic pathogenesis. CD4+ T cells and CD68+ macrophages were observed in inflammatory sacroiliac joints, entheses, and peripheral joint synovium from AS patients [27]. When innate immune cells overloaded to dissolve the bacterial intestinal translocation in IBD patients, the adaptive immune system was abnormally over-activated. The activation of Th1 and Th17 cells was dominant in patients with CD and activation of Th2 cells in patients with UC. CD68+ macrophages increased in inflamed mucosa from IBD patients, as compared to those in normal mucosa [28]. Anti-TNF agents, which can block the production of acute phase proteins and modify cell migration and proliferation, were used as the therapeutic agent in both IBD and AS.
Severe colitis in IBD patients with AS can be explained by leaky gut theory. High permeability of intestinal barrier is one of the characteristics observed in the bowel from IBD patients. The higher intestinal permeability was correlated with the higher severity of experimental colitis in the murine model [29-31]. The degree of colitis was influenced by the deficiency of structural proteins contributing to gut integrity or cytokines/chemokines related to intestinal inflammation and barrier function. The high intestinal permeability was also found in patients with AS and their first-degree relatives, meaning that abnormal intestinal permeability is likely to be genetically related [32]. Although the evidence of the relationship between enteric pathogen and AS was weak, the antigens of enteric pathogens, Yersinia enterocolitica and Salmonella enterica were identified in joints from patients with HLA-B27-associated reactive arthritis [33].
To our knowledge, this is the only study evaluating the characteristics and disease course of IBD patients with concomitant AS in Asia, where the prevalence of IBD and AS is increasing [34-36]. This refers to the fact that our study can help clinicians to set up treatment strategy for patients with IBD and AS. However, this study has some limitations. The number of patients with CD and AS was not enough to consider the irrelevance of accompanying AS to the severity of CD. The number of patients with both IBD and AS was too small to compare the differences between those diagnosed with IBD before the onset of AS and those diagnosed with AS before the onset of IBD. Because many medications, especially biologics, are effective to treat both IBD and AS, one specific medication used for AS treatment can change the disease activity of IBD, and vice versa.
In conclusion, IBD patients with concomitant AS had a higher risk of the biologics or small molecule therapies for IBD than those without AS. Based on our study, clinicians should pay more attention to IBD patients with concomitant AS.
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
Lee HJ and Kang HW are editorial board members of the journal but were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Data Availability Statement
Not applicable.
Author Contributions
Conceptualization: Yoon H, Koh SJ, Park JW, Kang HW. Formal analysis: Jun YK, Kim AH, Kim KW. Methodology: Yoon H, Koh SJ, Park JW, Kang HW. Project administration: Lee HJ, Im JP, Park YS, Kim JS. Writing - original draft: Jun YK. Writing - review & editing: Jun YK, Yoon H, Koh SJ. Approval of final manuscript: all authors.
Additional Contributions
The authors are grateful to Seulji Kim, Hosun Yu, Young Yi Choi, Jaewook Shin, and Minji Oh for their supports of SIRN research works.
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Table 1. Characteristics of Patients Diagnosed with IBD after the Onset of AS and Those before the Onset of AS
ir-2022-00057-suppl1.pdfSupplementary Table 2. Characteristics Patients with UC and CD after Propensity Score Matching
ir-2022-00057-suppl2.pdf