Diagnosis of inflammatory bowel disease–Asian perspectives: the results of a multinational web-based survey in the 8th Asian Organization for Crohn’s and Colitis meeting
Article information
Abstract
Background/Aims
Inflammatory bowel disease (IBD) is no longer a rare disease in Asia, thus it needs to prepare recommendations relevant to Asian patients. This study aimed to identify disparities in the process of the diagnosis of IBD in Asian countries/regions.
Methods
In line with the 2020 Asian Organization for Crohn’s and Colitis annual meeting, a multinational web-based survey about Asian physicians’ perspectives on IBD was conducted.
Results
A total of 384 Asian physicians (99 in China, 93 in Japan, 110 in Korea, and 82 in other Asian countries/regions) treating IBD patients from 24 countries/regions responded to the survey. Most respondents were gastroenterologists working in an academic teaching hospital. About half of them had more than 10 years of clinical experience in caring for patients with IBD. The European Crohn’s Colitis Organisation guideline was used most commonly for the diagnosis of IBD except for Japanese physicians who preferred their own national guideline. The Mayo score and Crohn’s Disease Activity Index were the most commonly used activity scoring systems for ulcerative colitis and Crohn’s disease, respectively. Endoscopy, not surprisingly, was the main investigation in assessing the extent and activity of IBD. On the other hand, there were disparities across countries/regions with regard to the favored modalities of small bowel and perianal evaluation of Crohn’s disease, as well as the use of serologic markers.
Conclusions
Results of the present survey revealed practical behaviors of Asian physicians in the diagnosis of IBD. Investigating the reasons for different diagnostic approaches among countries/regions might help us develop Asian guidelines further.
INTRODUCTION
The incidence of inflammatory bowel disease (IBD) in Asia has risen rapidly over the last few decades to become a global disease [1]. IBD is no longer regarded as a disease of only the Western world. Although the exact cause of the increase in IBD in Asia remains unclear, urbanization and industrialization are believed to be associated with the accelerating incidence of IBD [1,2]. Some highly urbanized Asian countries/regions such as Korea, Hong Kong, India, and Taiwan have reported at least a 2- to 3-fold increase in incidence rates of IBD [3-6]. The highest incidence rate of IBD in Asia was observed in India (9.31/100,000) [5].
The rapid increase in disease burden of IBD among Asian countries/regions might raise several concerns. First, the lack of consideration for the disease still exists among healthcare providers in Asia, which could lead to frequent underdiagnosis or misdiagnosis of IBD [7,8]. Second, there is growing evidence showing that clinical manifestations of IBD patients differ between the East and West [2,9,10]. Third, Asian countries/regions have relatively high background prevalence rates of infectious diseases mimicking IBD such as tuberculosis [11]. For these reasons, it may be unreasonable to apply Western practice recommendations to Asian countries/regions.
In an effort to identify variations in the practice pattern of different Asian countries/regions, the Korean Association for the Study of Intestinal Diseases (KASID) conducted a multinational survey for physicians who care for IBD patients in Asian countries/regions in the second annual meeting of the Asian Organization for Crohn’s and Colitis (AOCC) [12]. That survey revealed the vast differences in the approach of Asian physicians in diagnosing and managing IBD. These findings could be explained by the fact that there is no universal guideline for Asian patients with IBD, and most guidelines currently used for the management of IBD are from the West [13-17]. To solve this unmet need, the first Asian recommendations for IBD management have been developed by the AOCC and Asian Pacific Association of Gastroenterology recently, which take into account differences between the Asian and Western IBD patients [18].
The coronavirus disease 2019 (COVID-19) pandemic has led to an almost global lockdown, which has unexpectedly changed the 2020 AOCC annual meeting into a virtual congress. In spite of the restrictions in direct communication due to COVID-19, a follow-up online survey was planned to continue the exchange between Asian physicians and form a consensus on IBD care. The purpose of this study was to identify changes in terms of Asian physicians’ perceptions of IBD diagnosis.
METHODS
The first survey on Asian physicians’ perspectives of IBD was conducted in 2014 as one of the programs of the second annual meeting of AOCC [12]. For the follow-up research, KASID decided to conduct the second survey and present the results in the 8th annual meeting of AOCC which was held as a virtual congress in December 2020. The questionnaire for this research was developed in collaboration with the International Academic Exchange Committee, the Scientific Committee, and the IBD research group of KASID. It mainly consisted of 5 parts: personal information (8 items), diagnosis of IBD (17 items), treatment of IBD (33 items), infections in IBD (22 items), and vaccinations in IBD (15 items). Details of the questionnaire are shown in the Supplementary Material. Regarding the diagnosis of IBD, questions covered the following topics: diagnostic guidelines, classifications, assessing disease activity, endoscopic examination, serologic and stool test, and small bowel or perianal evaluation for Crohn’s disease (CD).
A web-based survey of a total of 95 questions was sent to about 16,000 multinational AOCC members with available email addresses using SurveyMonkey. Responses were collected online between September 16, 2020 and November 13, 2020. This study reports results of a subset of data regarding the diagnosis of IBD from the whole questionnaire. Results of other topics will be reported elsewhere. Because this study did not include any animal or human data and only report the results of web-based survey as in previous AOCC survey, ethical approval and informed consent to participants was not applicable.
RESULTS
1. Characteristics of Respondents
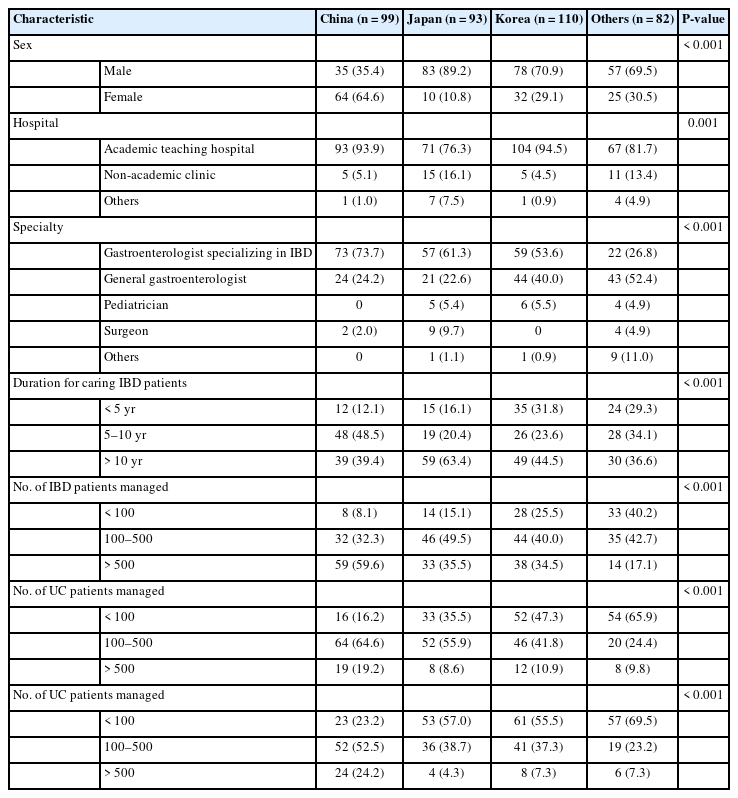
A total of 384 physicians from 24 countries/regions completed this survey. Respondents were from various Asian countries/regions (Korea 110, China 99, Japan 93, Taiwan 20, Hong Kong 10, Vietnam 8, Indonesia 7, India 6, Malaysia 6, Philippines 4, Pakistan 3, Australia 2, Bangladesh 2, Myanmar 2, Thailand 2, Turkey 2, Egypt 1, Iraq 1, Lebanon 1, Mongolia 1, New Zealand 1, Singapore 1, United Arab Emirates 1, and Uzbekistan 1). We divided them into 4 groups according to their nationality to facilitate comparative analyses (Table 1). Of these respondents, 65.9% (253/384) were males and 87.2% (335/384) were working in academic teaching hospitals. More than half (54.9%, 211/384) of these respondents were gastroenterologists specializing in IBD, followed by general gastroenterologists (34.4%, 132/384), pediatricians (3.9%, 15/384), and surgeons (3.9%, 15/384). Of these respondents, 46.1% (177/384) had more than 10 years of clinical experience of caring for patients with IBD and 40.9% (157/384) were managing between 100 and 500 IBD patients in their clinics.
2. Diagnostic Guidelines and the Montreal Classification
The European Crohn’s Colitis Organisation guidelines were the most commonly used guideline for the diagnosis of IBD (44.3%, 170/384), followed by the national diagnostic guideline of each country/region (37.8%, 145/384). For comparisons by country/region, only Japanese physicians preferred to use their national guideline (77.4%, 72/93) (Fig. 1A). More than half of the respondents answered that they always used the Montreal classification to classify their IBD patients. On the other hand, a significant number of Japanese physicians never used the Montreal classification (Fig. 1B and C).
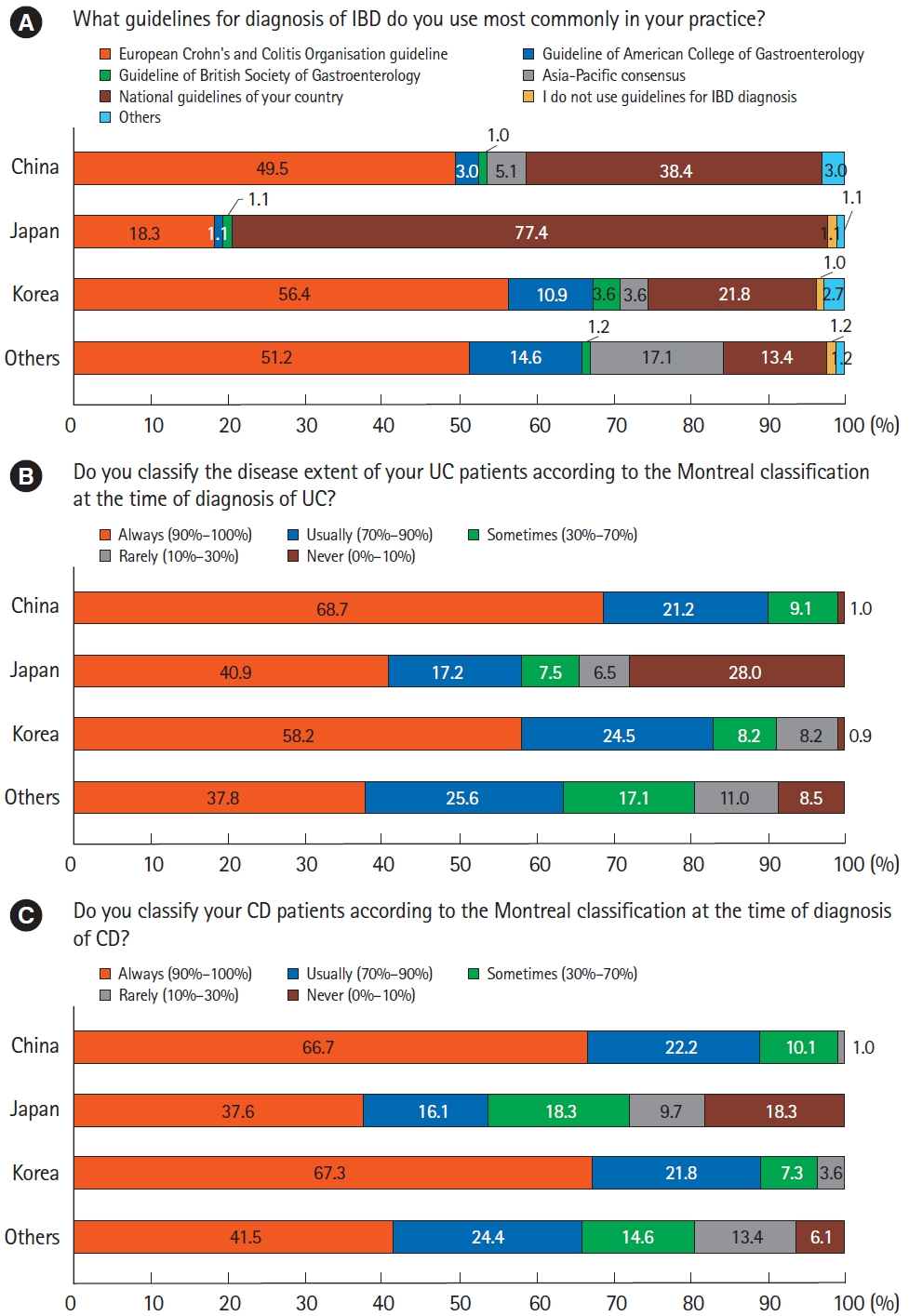
Diagnostic guidelines and the Montreal classification. (A) The most commonly used guidelines for the diagnosis of inflammatory bowel disease (IBD). (B) Use of the Montreal classification for classifying disease extent of ulcerative colitis (UC) at the time of diagnosis. (C) Use of the Montreal classification for classifying Crohn’s disease (CD) at the time of diagnosis.
3. Clinical, Endoscopic, and Radiologic Assessments of IBD
For clinical assessment of disease activity of ulcerative colitis (UC), the Mayo score was the most commonly used scoring system as expected (Fig. 2A). In the case of CD, the Crohn’s Disease Activity Index was the most chosen regardless of country or region (Fig. 2B). Of the respondents, 36.2% responded that fecal calprotectin was checked for IBD patients every 6 months and 26.3% responded that fecal calprotectin was checked every 3 months. On the other hand, 22.7% of respondents reported they did not check it at all, especially in Japan (36.6%) and other countries/regions (32.9%) (Fig. 2C).
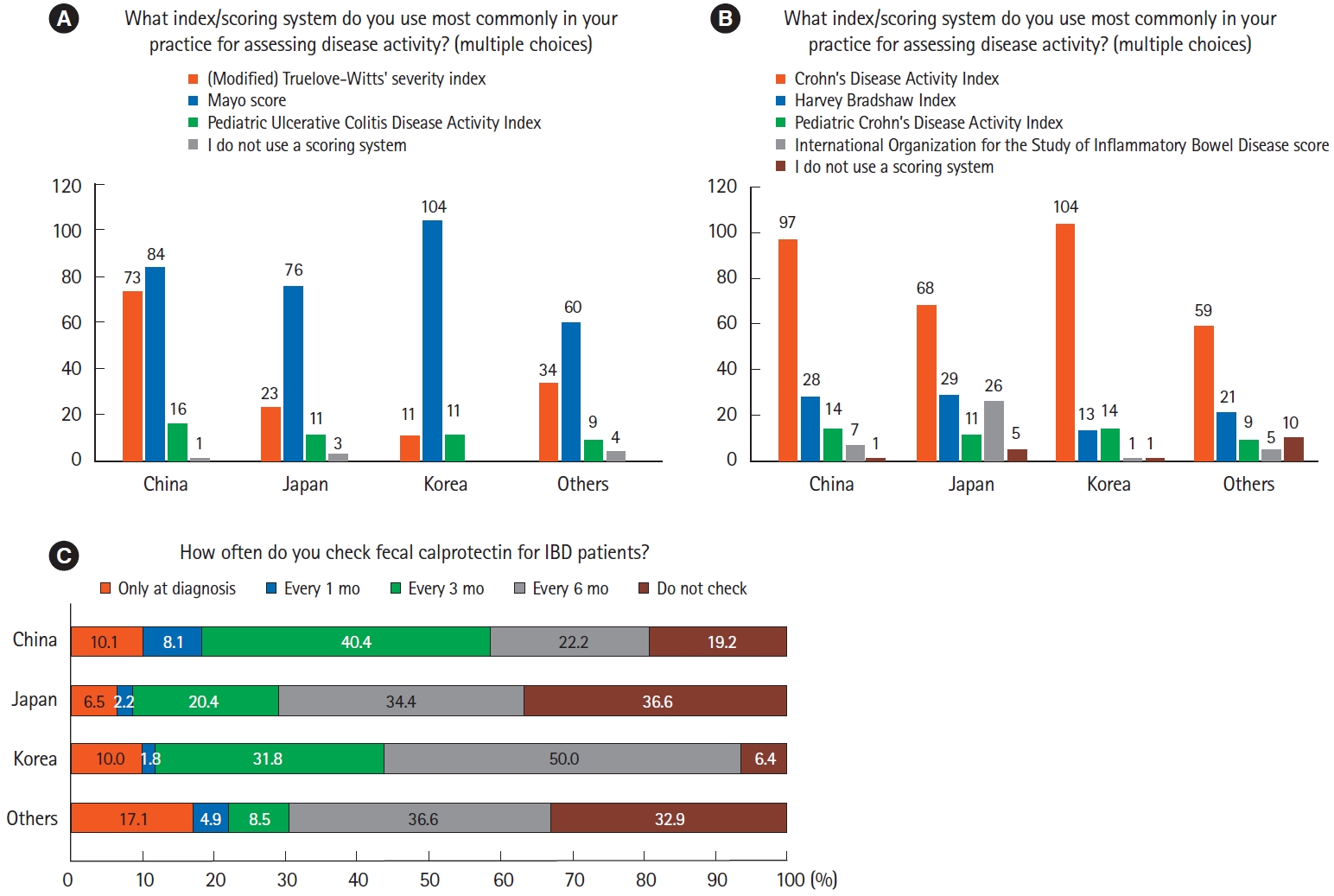
Clinical assessments. (A) Clinical scoring systems for ulcerative colitis. (B) Clinical scoring systems for Crohn’s disease. (C) Use of fecal calprotectin.
At the time of UC diagnosis, most respondents always (71.9%, 276/384) or usually (18.0%, 69/384) performed endoscopic examination to document activity and extent of the disease. Regarding the scoring system used for assessing the endoscopic severity, 64.6% (248/384), 7.3% (28/384), and 26.3% (101/384) of respondents used the Mayo endoscopic subscore (MES), the UC Endoscopic Index of Severity (UCEIS), and both, respectively. Only 1.8% (7/384) of respondents did not use a scoring system (Fig. 3A).
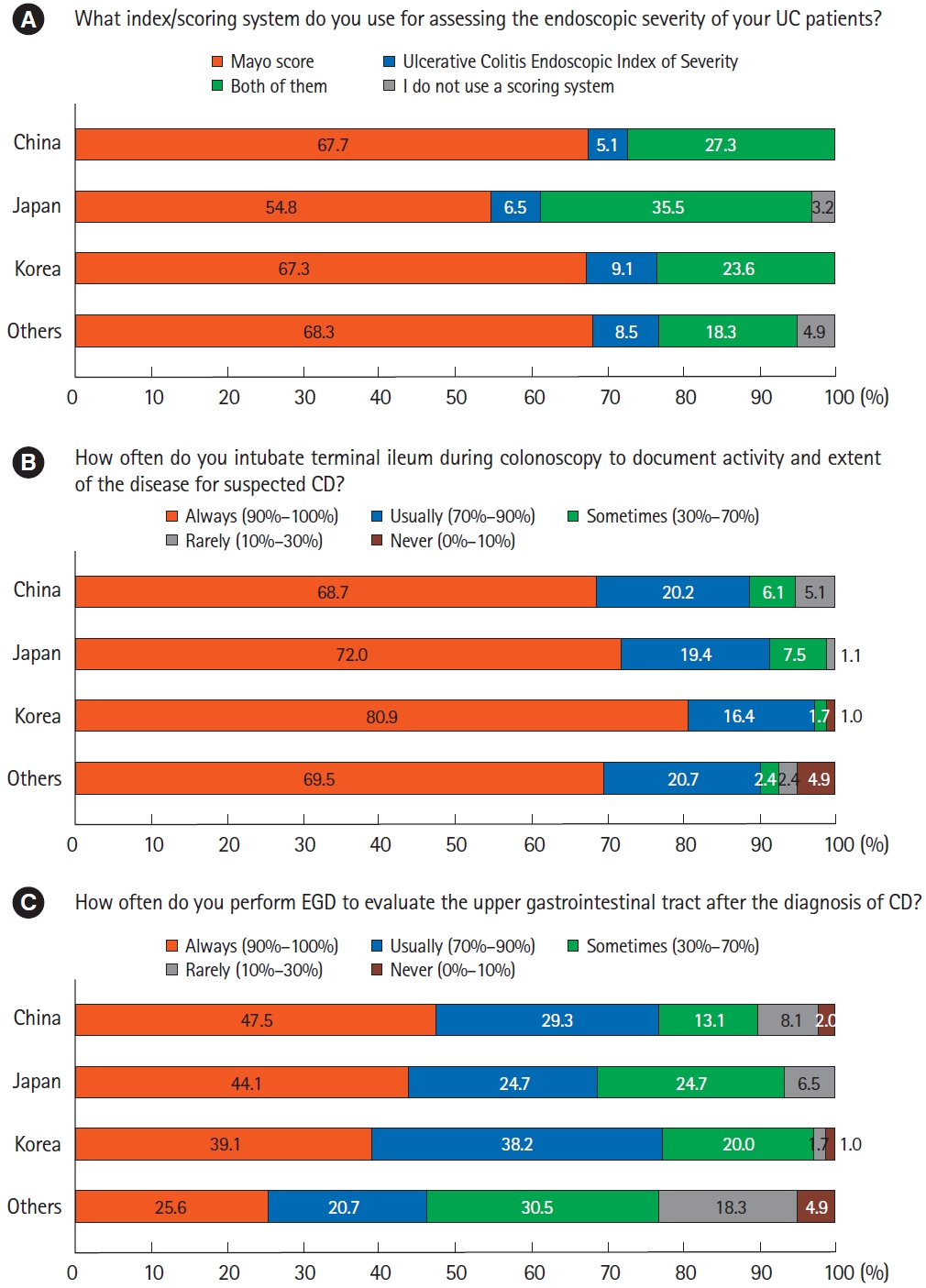
Endoscopic assessments. (A) Endoscopic scoring system for ulcerative colitis (UC). (B) Terminal ileum intubation during colonoscopy for Crohn’s disease (CD). (C) Esophagogastroduodenoscopy (EGD) for CD.
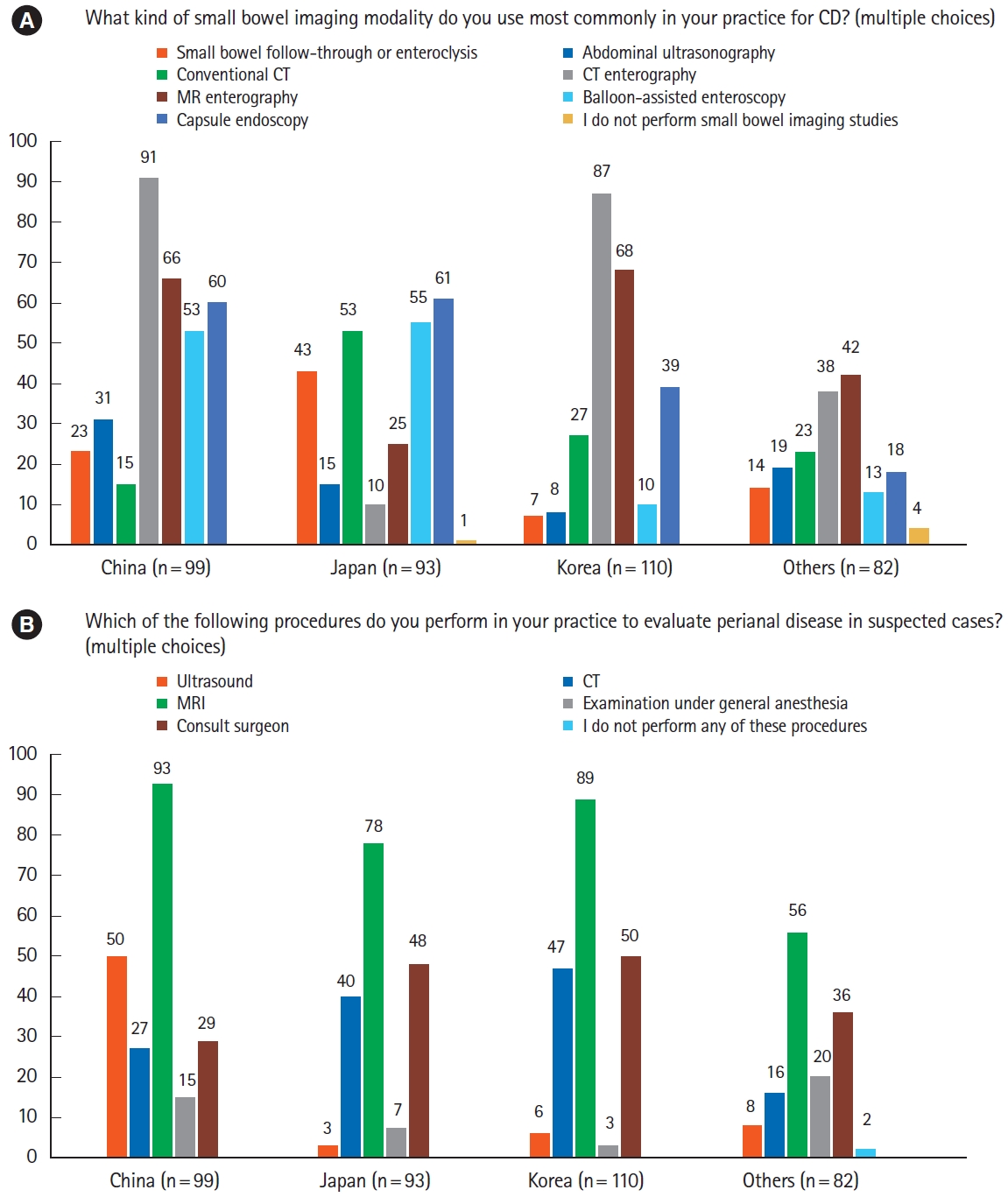
During colonoscopy of patients with suspected CD, the terminal ileum was almost always intubated to document activity and extent of the disease (Fig. 3B). Once CD was diagnosed, most respondents conducted esophagogastroduodenoscopy to evaluate upper gastrointestinal involvement of CD, which was somewhat less frequent for those from other countries/regions (Fig. 3C). There were differences between countries/regions in commonly used small bowel evaluation methods. Although respondents from China and Korea frequently used computed tomography (CT) or magnetic resonance enterography (MRE), those from Japan preferred conventional CT to CT or MRE (Fig. 4A). Small bowel follow-through was also frequently used method by Japanese doctors. Balloon enteroscopy was preferred by respondents from China and Japan over those from Korea and other countries/regions. For evaluation of perianal disease, pelvic magnetic resonance imaging was the most favored method in all countries/regions (Fig. 4B). In comparison, almost half of the respondents from China preferred the use of ultrasound.
4. Serologic Assessments and Exclusion of Infectious Diseases
Serologic tests including anti-neutrophil cytoplasmic antibody (ANCA) or anti-Saccharomyces cerevisiae antibody (ASCA) were not carried out by a considerable number of respondents from Japan and other countries/regions, whereas these were part of the diagnostic workup in more than 60% of Korean doctors (Fig. 5A and B).

Serologic assessments and exclusion of infectious diseases. (A) Anti-neutrophil cytoplasmic antibody (ANCA) and/or anti-Saccharomyces cerevisiae antibody (ASCA) for ulcerative colitis (UC). (B) ASCA and/or ANCA for Crohn’s disease (CD). (C) Microbiological culture for suspected UC. (D) Clostridium difficile test for suspected UC.
For suspected UC, about half or more respondents from China and Japan answered they always carried out microbiological culture to exclude infectious diseases, compared to only 14.5% and 26.8% from Korea and other countries/regions (Fig. 5C). Clostridioides difficile test was always performed by 28.0% to 47.5% of respondents (Fig. 5D).
DISCUSSION
This survey was conducted to investigate the current practice of Asian physicians on the diagnosis of IBD as part of the 8th annual meeting of AOCC in 2020. Our detailed questions about the diagnosis of IBD revealed differences across Asian countries/regions, especially about the diagnostic guideline and classification, the use of serologic markers, and the modality of small bowel and perianal evaluation of CD. Furthermore, since the previous survey conducted in the 2nd AOCC contained similar questions about the diagnosis of IBD, we could compare changes between the past and present care for IBD. The incidence of IBD has rapidly increased in Asian countries/regions during the past few decades due to Westernization and industrialization [1,2]. IBD is no longer a rare disease in Asia. There has been a growing interest in managing IBD patients among Asian physicians. In response to such interest, as many as 384 physicians from 24 countries/regions took part in this survey, which was increased compared to the previous survey perform ed in 2014 with 353 physicians from 10 countries/regions [12]. Of particular note, participation from other countries/regions besides China, Japan, and Korea has increased dramatically. This survey also demonstrated that Asian physicians have considerable experience in managing IBD patients. However, it must be noted that half of the correspondents are specialists in IBD and may not be representative of gastroenterologists in general. In addition, differences in terms of experience in caring for IBD patients or type of working hospitals across Asian countries/regions might be one of the factors affecting the overall results of the survey.
In terms of making the diagnosis of IBD, our survey showed that European Crohn’s Colitis Organisation and each country/region’s national guidelines have been mostly used. Montreal classification appears to be the main classification used except in Japan. This tendency was also observed in the previous survey in 2014. The trend of applying Asian specific criteria for the diagnosis and classification of IBD patients might be attributed to the differences in the clinical manifestation of IBD between the East and West [9]. Another reason could be that Asian countries/regions usually have a high prevalence of infectious colitis and tuberculosis that may mimic IBD [19]. Recently, the AOCC and the Asian Pacific Association of Gastroenterology have jointly proposed a practice guideline for Asian IBD patients to meet the growing demands of Asian physicians [18]. This guideline recommends pretreatment evaluation including differential diagnosis with intestinal tuberculosis (ITB), latent tuberculosis screening, and screening for opportunistic infections such as hepatitis B virus and hepatitis C virus.
The Mayo score and Crohn’s Disease Activity Index were dominantly used for evaluating disease activity by most Asian physicians. Compared to the previous survey conducted in 2014, twice as many Chinese doctors preferred the Truelove-Witts score over the Mayo in 2014, whereas in this survey, this trend was reversed [12]. The possible explanation for this change might be because the Mayo score requires endoscopic evaluation of the colonic mucosa to assess disease activity which could be a more objective scale than the Truelove-Witts score which is calculated only based on clinical and laboratory variables [20,21]. Fecal calprotectin is a good noninvasive biomarker for evaluating disease activity [22,23]. Our survey showed differences in the use of fecal calprotectin across countries/regions, which might be due to limited access to this biomarker in many Asian centers [9].
Results from our survey indicated that endoscopic examination for documenting the activity and extent of IBD is a universal practice in Asian countries/regions. In the case of UC, almost all respondents used the endoscopic scoring system, including UCEIS and MES. MES is easy to calculate. It is the most widely used scoring system in clinical trials. However, it is not validated and interobserver agreement can vary markedly [21,24]. UCEIS, unlike MES, is a validated endoscopic scoring system with an adequate interobserver agreement [25]. Although it is not widely used due to its unfamiliarity [26,27], it appears that more Asian physicians are adopting this validated score in their clinical practice.
We found that preferred modalities for evaluating small bowel and perianal lesion of CD varied significantly among countries/regions. For small bowel imaging, participants from China, Korea, and other countries/regions favored CT enterography and MRE, whereas Japanese physicians still preferred conventional CT and small bowel follow-through. More than half of respondents from China and Japan used balloon-assisted enteroscopy, although this modality was not favored by participants from Korea or other countries/regions. Despite these differences in small bowel evaluation modalities between Asian countries/regions, small bowel examinations are frequently performed due to the high prevalence of small bowel CD, which is estimated to be over 70%. 4 Since small bowel inflammation does not correlate well with clinical or biochemical markers, the importance of small bowel evaluation through small bowel endoscopy or cross-sectional imaging is increasingly emphasized [28]. For perianal imaging, all respondents preferred pelvic magnetic resonance the most. However, Chinese physicians also used ultrasound frequently. These differences could be explained by each country/region’s health care system such as the level of accessibility to radiologic equipment and various medical insurance systems.
Serologic markers including ANCA and ASCA could be used to support a diagnosis of IBD. Especially in Asian countries/regions where it is important to rule out other infectious colitis such as ITB, the addition of these serological antibodies to endoscopic examinations could play a role in helping physicians diagnose IBD more convincingly. Although Korean physicians mostly preferred serologic markers, a large number of Japanese and those from other countries/regions did not use them. It has been reported that the detection of ANCA and ASCA IgA is significantly lower in Chinese than in Caucasian patients [29]. The exact reason for this lower use of serologic markers by physicians in Japan and other countries/regions is unclear. The role of ASCA in differentiating CD from ITB is controversial; studies in East Asia suggest there may be a role, in contrast studies from India failed to show a benefit [30-33]. Another possible explanation for this result is thought to be that ASCA could not be covered by insurance reimbursement in Japan. Meanwhile, in other countries/regions, microbiological cultures and serological tests are not performed frequently, which could lead to misdiagnosis.
In conclusion, results of the present survey revealed the practice pattern of Asian physicians from diverse countries/regions in the diagnosis of IBD. Differences in some aspects of diagnostic approaches were also verified. It would be important to continue to make efforts to develop proper recommendations for Asian IBD patients who have unique disease features compared to Western IBD patients. Investigating the reasons for different diagnostic approaches among countries/regions might help us develop Asian guidelines for IBD in the future.
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
Lee HH and Ran ZH are editorial board members of the journal but were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Data Availability Statement
Not applicable.
Author Contributions
Conceptualization: Lee HH, Park JJ, Lee BI, Ran ZH. Data curation: Lee HH. Investigation: Lee HH. Methodology: Lee HH, Park JJ, Lee BI, Ran ZH. Supervision: Hilmi I, Sollano J. Writing - original draft: Lee HH. Writing - review & editing: Park JJ, Lee BI, Hilmi I, Sollano J, Ran ZH. Approval of final manuscript: all authors.
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Material
Detailed questions used in the current survey