Treatment of primary sclerosing cholangitis combined with inflammatory bowel disease
Article information
Abstract
Primary sclerosing cholangitis (PSC) is a progressive cholestatic, inflammatory, and fibrotic disease that is strongly associated with inflammatory bowel disease (IBD). PSC-IBD represents a unique disease entity and patients with this disease have an increased risk of malignancy development, such as colorectal cancer and cholangiocarcinoma. The pathogenesis of PSC-IBD involves genetic and environmental factors such as gut dysbiosis and bile acids alteration. However, despite the advancement of disease characteristics, no effective medical therapy has proven to have a significant impact on the prognosis of PSC. The treatment options for patients with PSC-IBD do not differ from those for patients with PSC alone. Potential candidate drugs have been developed based on the pathogenesis of PSC-IBD, such as those that target modulation of bile acids, inflammation, fibrosis, and gut dysbiosis. In this review, we summarize the current medical treatments for PSC-IBD and the status of new emerging therapeutic agents.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic and relapsing inflammatory disorder of the gastrointestinal tract that comprises ulcerative colitis (UC) and Crohn’s disease (CD). IBD is a global disease with a recent increasing incidence and prevalence in Asian countries [1-4], and Asian countries are classified into the acceleration in incidence of the epidemiological stages [5]. IBD patients require life-long treatment and may experience diverse extraintestinal manifestations throughout their lifetime [6-8].
Primary sclerosing cholangitis (PSC) is the most trouble-some hepatobiliary manifestation of IBD. PSC is a chronic progressive cholestatic, inflammatory and fibrotic disease, which is characterized by intrahepatic and extrahepatic biliary strictures [9,10]. The association between IBD and PSC is remarkable; in particular, UC is strongly associated with PSC, and up to 80% of patients with PSC also have UC [10,11]. The prevalence of PSC in IBD patients is approximately 8% in North America and Europe [12], whereas, the prevalence rates of PSC among Asian IBD patients are lower than those in Western countries and range from 0.39% to 2.3% [13-16]. This discrepancy may reflect the variations in disease genotypes and phenotypes between Asian and Western countries [17,18]. PSC-IBD patients appear to have a distinct phenotype from classical IBD, and features such as male predominance, rectal sparing, extensive colitis, and backwash ileitis are commonly observed [9,10,15]. The intestinal inflammation in PSC-IBD patients tends to have mild disease activity in endoscopy and also presents mild cryptitis on histologic examination [19]. Indeed, PSC-IBD patients have less aggressive clinical course of IBD than classical IBD patients, as indicated by reduced use of steroids, decreased hospitalizations and a less frequent need for immunosuppression [10,19,20]. However, the risk of malignancy, such as colorectal cancer and cholangiocarcinoma, is remarkably increased in PSC-IBD patients. Patients with PSC-IBD had a 4-fold higher risk of colorectal cancer, which is diagnosed at an earlier age and 28 times greater risk of cholangiocarcinoma compared to patients with IBD alone [21]. Therefore, PSC-IBD patients need strict surveillance and monitoring strategies for early detection of malignancy [21-25].
PSC and IBD can be diagnosed at various points in time. IBD may manifest prior to or at the same time as PSC. Additionally, PSC can be diagnosed after proctocolectomy and IBD may be apparent at a late stage of PSC [26]. In IBD patients, the early diagnosis of PSC is challenging. Concurrent PSC is usually asymptomatic or shows mild symptoms, such as fatigue. Usually, PSC can be diagnosed in longstanding IBD patients when biochemical screening reveals abnormal findings of liver chemistry, such as serum alkaline phosphatase (ALP). Therefore, if PSC is not suspected, it may be underdiagnosed in patients with IBD. Recent guidelines recommend that PSC should be considered in IBD patients when they show symptoms of cholestasis [25]. Moreover, IBD activity can be mild in patients with PSC, therefore, in newly diagnosed patients with PSC, ileocolonoscopy with biopsies should be performed to detect coexistent IBD early [25].
The exact pathogenesis of PSC-IBD remains poorly understood and appears to be complex. However, recent studies suggest that multiple factors are involved in the pathogenesis of PSC-IBD including genetic susceptibility, the innate and adaptive immune-mediated pathway, altered bile acids homeostasis, and gut dysbiosis [27-29]. The gut–liver axis plays an important role in the pathogenesis of PSC-IBD, just as gut inflammation influences the liver through immune cells, microbiota and modification of bile acids, which are derived from the liver and also affect the gut [30]. Although much progress has been made in understanding the pathogenesis of PSC-IBD, effective therapeutic options are still lacking. In this review, we summarize the current treatment modalities and introduce emerging potential pharmaceutical agents for patients with PSC-IBD (Fig. 1).

Therapeutic approach of primary sclerosing cholangitis (PSC) combined with inflammatory bowel disease (IBD). IBD management should be continued regardless of PSC treatment. Symptomatic care and management of complications of PSC are currently implemented, however, with the advancement of understanding of pathogenesis of PSC-IBD, several new therapeutic agents are developed and undergone the clinical trials. UDCA, ursodeoxycholic acid; TPL, transplantation; norUDCA, norursodeoxycholic acid; FXR, farnesoid X receptor; PPARs, peroxisome proliferator-activated receptors; ASBT, apical sodium-dependent bile acid transporter; LOXL2, lysyl oxidase-like 2; CCR2, C-C chemokine receptor 2; CCR5, C-C chemokine receptor 5; α4β7, alpha 4 beta 7; VAP-1, vascular adhesion protein 1; TNF, tumor necrosis factor.
CURRENT TREATMENT MODALITIES OF PSC-IBD
Currently, there are no proven medications for the treatment of PSC. Despite PSC being an immune-mediated disease, several immunosuppressants, including corticosteroids, methotrexate, and tacrolimus, failed to demonstrate effectiveness in the treatment of PSC [31,32]. Medical therapy is usually intended to manage symptoms such as pruritus and fatigue and complications of PSC. Liver transplantation (TPL) is required for patients with advanced PSC. At present, the treatment options for patients with PSC-IBD do not differ from the patients with PSC alone. Besides PSC treatment, patients with PSC-IBD should continue the management of intestinal inflammation. The survival of patients with PSC-IBD was significantly worse than that of patients with IBD-only (P=0.001) [33]. Moreover, the coexistence of IBD was the risk factor of PSC recurrence after liver TPL [34].
1. IBD Treatment
According to IBD treatment guidelines, the use of 5-aminosalicylic acid, steroids, thiopurines, and biologics such as anti-tumor necrosis factor (TNF) agents is recommended to control the activity of bowel inflammation in patients with PSC-IBD [35]. However, steroids and thiopurines do not improve the clinical course of combined PSC [36]. Anti-TNF agents are commonly used in patients with moderate to severe IBD. Whether patients with PSC-IBD respond to anti-TNF agents similarly to patients with IBD-only is uncertain. In addition, there is a concern regarding the risk of infectious complications, such as cholangitis, when treating patients with anti-TNF agents. In a small randomized controlled trial (RCT) that evaluated the use of infliximab for 6 months in patients with PSC (n=10), infliximab did not demonstrate efficacy without risk of infection [37]. The retrospective analysis of 141 patients with PSC-IBD with anti-TNF agents (infliximab or adalimumab) for 3 months revealed that 48% of patients responded to anti-TNF agents in IBD disease activity, suggesting an attenuated response compared with patients with IBD-only patients, in which the early response rate was reported as more than 60% [38,39]. Moreover, limited effects were observed on serum ALP level. At 3 months, the adalimumab group (n=23) showed a median 15% reduction in serum ALP level compared to base line (P=0.001), whereas infliximab group (n=66) showed a median 4% reduction in serum ALP level (P=0.306). At 12 months, the adalimumab group (n=14) had a 33% reduction in serum ALP level, although it was not significant (P=0.11). In terms of safety, anti-TNF agents did not exacerbate PSC symptoms or side effects such as cholangitis [40].
Another retrospective study also found that adalimumab (n=19) treatment showed a significant decrease in serum ALP after 6 to 8 months (P=0.03), but the decrease was not significant after 12 to 14 months (P=0.24). Infliximab treatment showed no improvement in serum ALP level [41]. Therefore, treatment with anti-TNF agents is effective in patients with PSC-IBD in terms of IBD activity control, but the response rates are decreased compared to those in patients with IBD-only. Evidence on the effects of anti-TNF agents on combined PSC is limited, and only adalimumab shows initial response; however, sample size is too small to draw conclusions. Moreover, considering the retrospective study design, future prospective studies on the effect of adalimumab on PSC-IBD are needed to elucidate its mechanism of action. In terms of safety, the use of thiopurines or anti-TNF agents has no harmful effects on the risk of infection or clinical outcomes of PSC-IBD [37,40-42].
Vedolizumab is a selective IgG1 monoclonal antibody that binds to α4β7 integrin, which is expressed on intestinal lymphocytes. Vedolizumab inhibits the α4β7 integrin–mucosal addressin cellular adhesion molecule-1 (MAdCAM-1) interaction, which blocks lymphocyte trafficking to inflammation site of the gastrointestinal tract [43]. Vedolizumab is currently used as an effective treatment modality for IBD [44,45]. Considering the mechanism of action of the drug and the over-expression of MAdCAM-1 in the hepatic endothelial cells of PSC, vedolizumab was expected to be a potential candidate for the treatment of PSC-IBD. In a large retrospective study by the International PSC Study Group, 102 patients with PSC-IBD who received at least 3 doses of vedolizumab were evaluated [46]. Of these patients, 56.8% showed endoscopic improvement of IBD, whereas, only 20.6% of them showed serum ALP reduction by 20% or more. Additionally, 20.6% of patients were accompanied by liver-related adverse events [46].
Another retrospective study of patients with PSC-IBD who were treated with vedolizumab (n=75) showed that only 4 patients (11%) had benefited from as reduction of serum ALP at 54 weeks posttreatment, indicating a lack of effectiveness of vedolizumab on liver function in patients with PSC-IBD [47]. Therefore, despite the significant effectiveness of vedolizumab on the treatment of IBD, the improvement of concomitant PSC was limited. As such, a phase III trial using vedolizumab in patients with PSC-IBD was withdrawn (NCT03035058) [48].
2. Ursodeoxycholic Acid
The most widely used drug in the treatment of PSC is ursodeoxycholic acid (UDCA). The mechanism of action of UDCA includes stimulation of hepatocellular and cholangiocellular secretion, anti-apoptosis and reduction of bile acids toxicity [49]. However, the exact mechanism of UDCA still has uncertainity [50].
Biochemical improvements were demonstrated after treatment of low dose UDCA (13–15 mg/kg/day) in patients with PSC, however, there were no differences in time to treatment failure or time to liver TPL between UDCA and placebo [51,52]. In addition, treatment of moderate dose UDCA (17–23 mg/kg/day) for a longer period (5 years) failed to reduce mortality or liver TPL rate [53]. Another RCT with high dose UDCA (28–30 mg/kg/day) also failed to demonstrate any benefits but showed higher rates of serious adverse events [54]. Therefore, recent UK-PSC treatment guideline recommends against the routine use of UDCA for newly diagnosed PSC patients [55]. However, use of UDCA at low or moderate dose is still prevalent in clinical practice in many countries. In addition, UDCA withdrawal for 3 months caused a significant worsening of liver chemistry [56]. The American Association for the Study of Liver Diseases practice guidance suggests that patients with a persistently elevated serum ALP can be considered for UDCA treatment at 13–23 mg/kg/day [25]. Nevertheless, considering limited evidence of UDCA in long-term outcomes of PSC, effective medical therapy is still unmet need and novel therapy is necessary.
3. Endoscopic Intervention
PSC can lead to significant bile duct stenosis at all levels in the biliary tree. Dominant strictures are present in up to 45% of PSC patients and if accompanied with impaired bile flows and worsening cholestasis, endoscopic intervention is required with balloon dilatation or stent insertion to relieve the dominant strictures [57]. Balloon dilatation is generally preferred to stent insertion given similar efficacy and lower complications such as pancreatitis, cholangitis, and bacteremia [58]. In cases of combined cholangitis, treatment with antibiotics should be initiated [59].
4. Liver TPL
Advanced liver disease attributed to PSC is a well-established indication for liver TPL. Indeed, liver TPL is the most reliable and effective treatment of PSC and is the fifth most common indication for total adult liver TPL in the United States [60]. Treatment guideline recommended patients with PSC should be referred for consideration of liver TPL if there are any liver-related complications [55]. The outcomes of PSC-related liver TPL are excellent with 5-year survival rates of 80% to 85% [61]. However, even after liver TPL, there is a risk for PSC recurrence. PSC recurrence was reported about 20% and the coexistence of IBD was the risk factor of PSC recurrence (hazard ratio [HR], 1.73) [34]. Further, active IBD at the time of liver TPL is presumed as a significant risk factor of graft failure (HR, 10; P=0.001) [10,62]. Therefore, patients with PSC-IBD should be maintained in remission state of IBD activity by the time of liver TPL to improve the outcome [62].
NEW THERAPEUTIC AGENTS
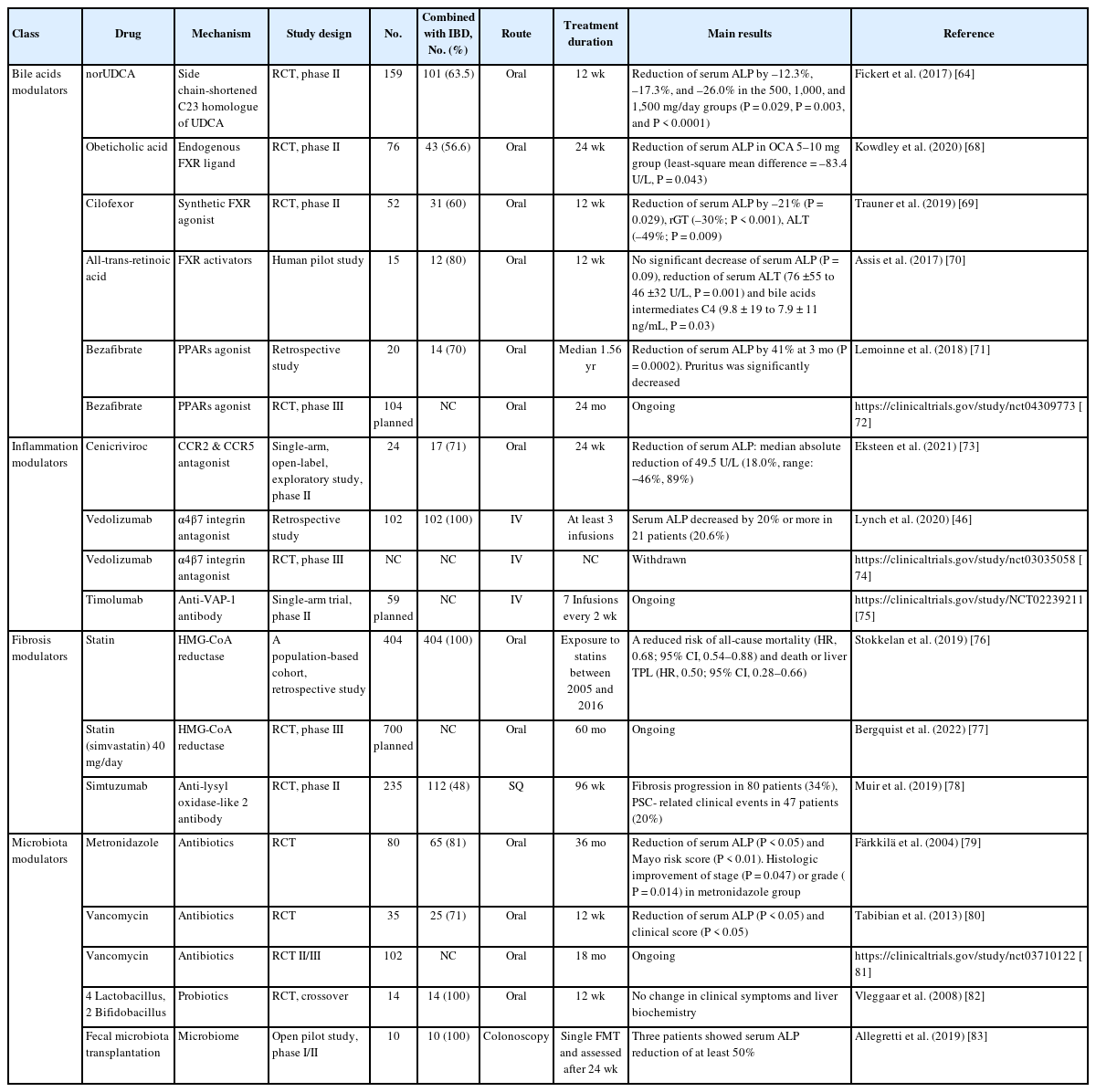
Understanding the pathogenesis of PSC-IBD is essential for identifying new therapeutic modalities, and providing opportunities for developing new therapeutic agents [48,49,63]. Several potential candidate drugs are undergoing clinical trials (Table 1). Most clinical trials of new therapeutic agents include more than 50% of concomitant IBD patients.
1. Bile Acids Modulators
PSC is one of cholestatic liver disease which is characterized by accumulation of toxic bile acids in liver. Altered bile acids composition is also regarded as an important pathogenetic contributor. Considering the toxic effects of bile acids on cholangiocytes, several potential drugs targeting bile acids modulation are developed to improve the cholestasis, increase the bicarbonate secretion and bile acids regulation [48,49,64].
1) Norursodeoxycholic Acid
New derivative of UDCA, 24-norursodeoxycholic acid (norUDCA) is a side chain shortened C23 homologue of UDCA. norUDCA is able to undergo cholehepatic shunting and stimulate bicarbonate secretion by cholangiocytes [65]. In animal models, norUDCA has shown remarkable anti-cholestatic, anti-inflammatory, anti-fibrotic, and anti-proliferative effects [65,66], suggesting it may be effective in cholestatic liver disease including PSC. A phase II RCT was conducted to evaluate the safety and efficacy of norUDCA (500, 1,000, or 1,500 mg/day) in patients with PSC. Treatment for 12 weeks showed significant reductions of serum ALP in a dose-dependent manner with an excellent safety profile [64]. These therapeutic effects are not associated with concomitant IBD presence. In addition, norUDCA had no effect on IBD activity. A larger phase III study of long-term efficacy of norUDCA 1,500 mg/day is ongoing.
2) Farnesoid X Receptor Agonists
Other bile acids related agents are farnesoid X receptor (FXR) agonists such as endogenous FXR ligands or nonsteroidal FXR agonists. FXR, a nuclear receptor for signaling of bile acids, plays an important role in bile acids homeostasis. Obeticholic acid (OCA), a native FXR ligand has been shown to regulate bile acids transport as well as possessing anti-inflammatory and anti-fibrotic effects [67]. A phase II RCT with high dose of OCA (5–10 mg/day) in patients with PSC revealed a significant decrease of serum ALP compared to placebo after 24 weeks treatment (P=0.043), although a higher rate of pruritus was observed in the OCA group [68]. Regarding concomitant UC, no clear treatment or dose-related differences were observed. A majority of patients with UC had remission state at enrollment and maintained the remission state to week 24.
Cilofexor is a synthetic FXR agonist which has shown antiinflammatory and anti-fibrotic effects. In a phase II RCT, cilofexor 100 mg/day for 12 weeks treatment in patients with PSC decreased serum ALP with tolerable safety (P=0.029) [69]. All patients with IBD had quiescent disease at enrollment and no patient had exacerbation of IBD activity during treatment. A phase III RCT with cilofexor is currently ongoing.
All-trans-retinoic acid is an indirect activator of the FXR transcription complex. A pilot study was conducted with combination therapy of all-trans-retinoic acid (45 mg/m2/day) and UDCA for 12 weeks. Although there was no significant decrease of serum ALP (P=0.09), significant reduction of alanine transaminase and the bile acids intermediates were observed [70].
3) Peroxisome Proliferator-Activated Receptors Agonists
Peroxisome proliferator-activated receptors (PPARs) play a critical role in the regulation of bile acids homeostasis via increase of biliary phospholipid secretion, bile acids detoxification, and inhibition of bile acids synthesis [84-86]. PPARs also have anti-inflammatory effects through reduction in nuclear factor-κB dependent gene activation and inflammatory cytokines as well as anti-fibrotic effects [87]. Bezafibrate is an agonist of PPARα/γ and the efficacy of bezafibrate in combination with UDCA has been demonstrated in patients with primary biliary cholangitis, another cholestatic liver disease [88]. In a retrospective study, 20 PSC patients were treated with bezafibrate (400 mg/day) or fenofibrate (200 mg/day) combined with UDCA and showed a reduction of serum ALP by 41% at 3 months (P=0.0002) [71]. Of the 14 concomitant IBD patients, 1 patient experienced IBD flare and discontinued the fibrate. A phase III RCT with bezafibrate is currently ongoing for the PSC patients with persistent cholestasis despite standard UDCA therapy.
4) Apical Sodium-Dependent Transporter Inhibitors
Several inhibitors of apical sodium-dependent bile acid transporter (ASBT) are also ongoing in clinical trials [48]. Inhibition of ASBT blocks ileal bile acids reuptake, and reduces the amount of enterohepatic circulation of bile acids. Currently, ASBT inhibitors are considered promising candidate drugs for cholestatic disease including PSC.
2. Inflammation Modulators
Through genetic studies, PSC is recognized as the immuneme-diated disease. In fact, both innate and adaptive immune pathway are involved in the pathogenesis of PSC-IBD [89-91].
1) C-C Chemokine Receptor 2/5 Antagonists
Monocyte chemotactic protein-1 is overexpressed in PSC patients in accordance with dysregulated innate immunity [92]. When hepatobiliary tract receives the injuries, C-C chemokine receptor (CCR) 2 and CCR5, the main receptors of monocyte chemotactic protein-1, are rapidly activated and induce macrophage and monocytes infiltration, hepatic stellate cells (HSC) activation, leading to hepatic inflammation and fibrogenesis [92,93]. In addition, CCR5 is highly expressed in active IBD and the lymphocyte grade has a positive correlation with CCR5 expression, suggesting the possibility of new therapies of CCR5 antagonist for IBD [94]. Cenicriviroc, a novel antagonist of CCR2 and CCR5, underwent a phase II open-label trial, and only showed a limited reduction (median 18%) of serum ALP after 24 weeks treatment [73]. Concomitant IBD patients were remission state (n=15) or had mild disease activity (n=4). During 24 weeks, no IBD flare was reported. Despite cenicriviroc not achieving the primary endpoint, it is still suggested to be a possible candidate drug for PSC, although further study is necessary to define the optimal dose.
2) Anti-Vascular Adhesion Protein Antibody
Timolumab is a monoclonal anti-vascular adhesion protein1 antibody. Vascular adhesion protein-1 is an adhesion molecule expressed by hepatic endothelial cells, which enables activated T cells to bind to hepatic endothelium [95]. The action of mechanism of timolumab is reduction of leukocyte trafficking to site of inflammation. A phase II single-arm trial of timolumab is ongoing with the primary endpoint of the decrease of serum ALP from baseline [48].
3. Fibrosis Modulators
PSC is a progressive inflammatory disorder causing activation of HSC and ultimately lead to the peribiliary fibrosis. Therefore, anti-fibrotic mechanisms are important potential target for PSC treatment [96].
1) Statins
Statins administration displayed clinical benefits to liver disease patients through anti-inflammatory/fibrotic effects in addition to cholesterol lowering effects [97]. In a Swedish PSC-IBD cohorts (n=2,914), 13.9% of patients were exposure to statins and statins use reduced death or liver TPL (HR, 0.50), suggesting an association between treatment with statins and improved outcomes in PSC-IBD patients [76]. Based on these results, a RCT is currently underway to evaluate the long-term efficacy of simvastatin on the prognosis in patients with PSC (NCT04133792) [77].
2) Anti-Lysyl Oxidase-like 2 Antibody
Simtuzumab, a monoclonal antibody targeting lysyl oxidase-like 2 (LOXL2) showed promising anti-fibrotic efficacy in animal model [98]. LOXL2 maintains the fibrogenic process through stabilization of the fibrotic matrix and promotes fibrogenic HSC. Along this line, elevation of LOXL2 in the serum and liver tissue of patients with PSC has been observed [99]. However, simtuzumab challenging for 96 weeks in patients with PSC failed to show significant anti-fibrotic benefits compared with placebo group in a phase IIb RCT [78]. Of the 112 concomitant IBD patients, 8 patients experienced IBD flare during treatment: 3 in simtuzumab group and 5 in placebo.
4. Microbiota Modulators
Disrupted intestinal barrier due to IBD can cause the influx of gut microbiota to hepatic circulation, leads to the hepatic inflammation of PSC. The involvement of the gut microbiota is regarded as a major contributing factor to development of PSC-IBD [27,30,100]. In the animal model, the epithelial barrier was disrupted by PSC-IBD patients-derived Klebsiella pneumonia (pathobionts) and subsequent bacterial translocation and IL-17-producing T helper 17-mediated hepatobiliary injuries were demonstrated [100]. Of interest, antibiotic treatment improved the T helper 17 immune response induced by K. pneumonia [100]. Furthermore, the presence of K. pneumonia were associated with reduced liver TPL free survival in patients with PSC-IBD [101].
Gut dysbiosis is reported in patients with PSC-IBD, that is, reduced bacterial diversity and a different global microbial composition are observed [102]. The dysbiosis of PSC-IBD is similar to those of PSC-only patients rather than IBD-only patients [103]. The current evidence provides a strong rationale for clinical trials of microbiota modulators that target the dysbiosis in PSCIBD patients.
1) Antibiotics
Gut dysbiosis perturbates the gut immune system and induces biliary injury via bile acids dysregulation as well as augmentation of toxin trafficking. Resolving the dysbiosis would be the key to treating of PSC. In fact, modulating microbiota using antibiotics showed promising results in clinical trials. An RCT using metronidazole (600–800 mg/day) and co-treatment with UDCA was evaluated in 80 PSC patients [79]. After 30 months, serum ALP (P<0.05) and New Mayo PSC Risk score (P<0.01) decreased markedly in the metronidazole group (P<0.05). Nonetheless, no significant effects on disease progression were found [79].
Vancomycin is a potent antibiotic against Gram-positive bacteria and is poorly absorbed, remains as high concentrations in the intestine. The RCT of oral vancomycin showed promising results in patients with PSC. Vancomycin (125–250 mg four times/day) and metronidazole were treated for 12 weeks and vancomycin group experienced significant reductions of serum ALP (P<0.05) and Mayo PSC risk score (P<0.05) with less adverse effects than metronidazole group [80]. Furthermore, a meta-analysis of 124 PSC patients who treated with antibiotics showed a significant reduction of serum ALP (65.6%, P<0.002) as well as severity of cholestasis [104]. Vancomycin may aid improving PSC either via direct effects on the microbiome or via host-mediated mechanisms [104]. A phase III RCT is undergoing to evaluate the efficacy of vancomycin in patients with PSC-IBD (NCT03710122).
2) Probiotics
Probiotics are live microorganisms which are expected to confer benefits to the host by regulation of intestinal microecology. Notably, each microorganism or combination of microorganisms has a distinct therapeutic potential in a diverse disease setting. There are only few studies examining the therapeutic potential of probiotics on patients with PSC-IBD (remission state of IBD activity). In a RCT of 14 PSC-IBD patients, probiotics having 4 Lactobacillus and 2 Bifidobacillus strains was tried for 3 months, however, no improvement in clinical symptoms (fatigue, pruritus, or frequency of stools) (P=0.89) as well as serum ALP level (P=0.99) were observed [82]. One patient in placebo group experienced IBD flare and improved by steroid treatment.
3) Fecal Microbiota TPL
Fecal microbial transplantation (FMT) is used to restore a healthy gut microbial environment. FMT is widely accepted as a reliable treatment option for recurrent or refractory Clostridioides difficile infection. Moreover, numerous trials have been performed to evaluate the efficacy and safety in various diseases including IBD and hepatic encephalopathy [105]. In an open pilot study of FMT in 10 PSC-IBD patients (7 patients in remission, 3 patients in mild activity) [83], 3 patients showed serum ALP reduction of at least 50% after 24 weeks of FMT with a good safety profile. Interestingly, an increase in bacterial diversity and engraftment in post-FMT patients correlated with decreased serum ALP levels (P=0.02) [83], implying the functional involvement of dysbiosis in biliary pathology. There was no flare of IBD activity. Further studies are needed for assessing long-term efficacy of FMT in PSC-IBD patients along with the careful observation of IBD disease activity.
CONCLUSION
Currently, there are no proven treatments that affect the clinical course of PSC-IBD. Despite PSC-IBD is classified as an immune-mediated disease, immunosuppressant treatments have proven to be ineffective. Additionally, the treatment modalities for IBD including anti-TNF agents have also limited effects on PSC prognosis, however, the safety seems acceptable. Basically, the treatment options for patients with PSC-IBD do not differ from those for patients with PSC alone. However, the increased risk of malignancy in patients with PSC-IBD needs the careful surveillance and monitoring strategies. Currently, UDCA is the most widely used drug; however, the role of UDCA in the treatment of PSC remains controversial.
Nonetheless, there are various clinical/preclinical investigation including modulation of bile acids, inflammation, fibrosis and microbiome based on the advanced understating of pathogenesis of PSC-IBD. In particular, bile acids modulators appear to be promising agents and have safety profile in terms of IBD activity. Combination therapy may be probably needed to achieve clinical efficacy.
Notes
Funding Source
Funding was provided by NIH grant 1R01CA258449 and PLRC Pilot & Feasibility grant PF 2019-05 to Hurley EH and Ko S through 1P30DK120531-01 to the Pittsburgh Liver Research Center.
Conflict of Interest
Kim YS is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Data Availability Statement
Not applicable.
Author Contribution
Conceptualization: Kim YS, Ko S. Supervision: Ko S. Validation: Hurley EH, Park Y, Ko S. Writing – original draft: Kim YS, Ko S. Writing – review & editing: Kim YS, Hurley EH, Park Y, Ko S. Approval of final manuscript: all authors.