Pharmacologic Agents for Chronic Diarrhea
Article information
Abstract
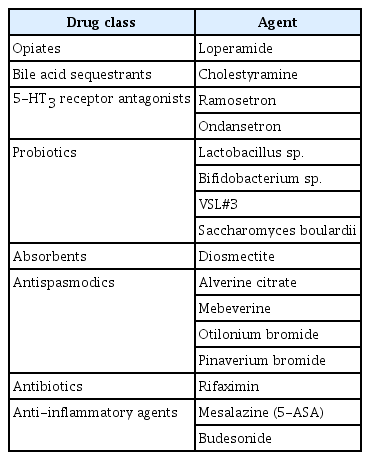
Chronic diarrhea is usually associated with a number of non-infectious causes. When definitive treatment is unavailable, symptomatic drug therapy is indicated. Pharmacologic agents for chronic diarrhea include loperamide, 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists, diosmectite, cholestyramine, probiotics, antispasmodics, rifaximin, and anti-inflammatory agents. Loperamide, a synthetic opiate agonist, decreases peristaltic activity and inhibits secretion, resulting in the reduction of fluid and electrolyte loss and an increase in stool consistency. Cholestyramine is a bile acid sequestrant that is generally considered as the first-line treatment for bile acid diarrhea. 5-HT3 receptor antagonists have significant benefits in patients with irritable bowel syndrome (IBS) with diarrhea. Ramosetron improves stool consistency as well as global IBS symptoms. Probiotics may have a role in the prevention of antibiotic-associated diarrhea. However, data on the role of probiotics in the treatment of chronic diarrhea are lacking. Diosmectite, an absorbent, can be used for the treatment of chronic functional diarrhea, radiation-induced diarrhea, and chemotherapy-induced diarrhea. Antispasmodics including alverine citrate, mebeverine, otilonium bromide, and pinaverium bromide are used for relieving diarrheal symptoms and abdominal pain. Rifaximin can be effective for chronic diarrhea associated with IBS and small intestinal bacterial overgrowth. Budesonide is effective in both lymphocytic colitis and collagenous colitis. The efficacy of mesalazine in microscopic colitis is weak or remains uncertain. Considering their mechanisms of action, these agents should be prescribed properly.
INTRODUCTION
Diarrhea is defined as the condition of having at least three loose or liquid stools each day. Diarrhea is not a disease, but a symptom and may be associated with diverse conditions. Acute diarrhea is defined as the abrupt onset of three or more loose stools per day that lasts no longer than 14 days. Chronic diarrhea is generally defined as a decrease in fecal consistency lasting longer than 4 weeks. An infection of the intestine is known to be the most common cause of acute diarrhea. Conservative treatments and antibiotics, if indicated, are the main therapy for this kind of diarrheal disease. Chronic diarrhea is usually associated with a number of non-infectious causes, including medications, IBS, IBD, hyperthyroidism, pancreatic insufficiency, and small bowel malabsorption. Since different etiologies and management are involved, diagnostic evaluation is required for chronic diarrhea. When definitive treatment is unavailable for chronic diarrhea, symptomatic drug therapy is indicated.
Although symptomatic treatment for diarrhea is practically necessary, particularly for chronic diarrhea, available drugs are limited and frequently not satisfactory. In this review, the available data on the pharmacologic agents for chronic diarrhea are summarized.
OPIATES
Loperamide is a synthetic opiate agonist for the µ receptors in the myenteric plexus of the intestinal wall.12 It is approved for the control of diarrhea symptoms in adults and children over 12 years of age. Loperamide inhibits release of acetylcholine through activation of µ receptors, resulting in decreased peristaltic activity.34 Furthermore, inhibition of acetylcholine release by loperamide leads to antisecretory activity, because muscarinic acetylcholine receptors exit on secretory epithelial cells in the gut wall. Therefore, loperamide reduces fluid and electrolyte loss, decreases fecal volume, and increases stool consistency.345
Loperamide is rapidly absorbed from the gastrointestinal tract, and metabolized in the liver. Its maximum effect occurs at 16-24 hours after administration. Absorbed loperamide is mainly excreted in the bile. The amount of loperamide entering the systemic circulation is not significant, indicating fewer systemic side effects. In addition, loperamide does not cross the blood-brain barrier well, suggesting no central side effects or risk of dependence.3 It has no analgesic effect and is not used for abdominal pain. Tolerance to the antidiarrheal effects of loperamide was reported in animal studies.6 However, loperamide has been used for the treatment of chronic diarrhea without evidence of tolerance in humans.7 Initial dosing is usually 2 mg twice a day.7 The effective dose varies widely. Increase of the dose is necessary if it is not effective in controlling diarrhea. The maximum daily dose is 16 mg. In general, loperamide is not recommended if an inflammatory condition of the bowel is suspected (visible blood in stools, dysentery, or acute colitis). It can be used for the treatment of infectious diarrhea without dysentery, but caution is advised.
BILE ACID SEQUESTRANTS
High levels of bile acids in the colon may cause diarrhea by increasing intestinal motility and secretion of mucus.8 Bile acid malabsorption can develop in patients with disease of the terminal ileum, ileal resection, or radiation injury. However, it may result from cholecystectomy, bacterial overgrowth, and intestinal dysmotility. Furthermore, bile acid malabsorption is also observed in some patients with functional diarrhea or IBS with diarrhea (IBS-D).9
Cholestyramine is a bile acid sequestrant that is generally considered as the first-line treatment for bile acid diarrhea. However, patient compliance is not good because of poor palatability.10 This drug consists of non-digestible resins that bind to bile acids in the intestine, and increase excretion of bile acids in the feces. Cholestyramine can be used for the symptomatic control of bile acid-induced diarrhea due to short bowel syndrome. It is also effective in patients with IBS-D, particularly those with elevated fecal bile acid excretion.11 Cholestyramine is usually given at an initial dose of 4 g/day, increased as needed to 4 g 2-4 times/day. In general, 4 g twice/day is effective in relieving diarrhea associated with bile acid malabsorption. Adverse effects may include constipation, nausea, bloating, flatulence, and abdominal pain. Other bile acid sequestrants such as colestipol and colesevelam are available for clinical use in some countries.12
5-HT3 RECEPTOR ANTAGONISTS
Studies suggest that serotonin mediates diarrhea and plays a role in IBS-D.13 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists reduce visceral sensation, slow colonic transit, and decrease the contractile and tonic responses to meal ingestion.1415 Previous trials and meta-analyses demonstrated that 5-HT3 receptor antagonists have significant benefits in patients with IBS-D.1617 Alosetron and cilansetron were developed as potent and specific. These agents have proven effective in IBS-D, but have serious side effects. Because of adverse effects of ischemic colitis and complications of constipation, alosetron is currently available with a very restricted indication in some countries, and cilansetron was never marketed.18
The 5-HT3 receptor antagonist, ramosetron, has been used in Japan since 1996 as an antiemetic for patients with cancer. A low dose of ramosetron in patients with IBS-D demonstrated its efficacy in clinical trials in Japan and South Korea.1920 In those trials, ramosetron 5 µg once daily improved stool consistency as well as global IBS symptoms. Data show that ramosetron has a lower risk of serious side effects than other drugs of the same class.21 Ondansetron, a selective 5-HT3 receptor antagonist developed in the mid-1980s, has been used as an antiemetic for controlling postoperative and chemotherapy-induced nausea and vomiting. Ondansetron has a long and excellent safety record, and a recent study demonstrated that it improved stool consistency, reduced stool frequency, and slowed colonic transit in IBS-D.
PROBIOTICS
Probiotics are live microorganisms, which may provide beneficial effects for the host when ingested. Their efficacy depends on the strain, dose, and viability of the microorganisms contained in the preparations. Probiotics including species of Lactobacillus and Bifidobacterium, VSL#3 (a combination of Bifidobacterium, Lactobacillus, and Streptococcus species), and Saccharomyces boulardii are commonly used.
Probiotics may have a role in the prevention of antibiotic-associated diarrhea.23242526 A recent meta-analysis confirmed that S. boulardii was effective in reducing the risk of antibiotic-associated diarrhea.27 However some studies failed to confirm the beneficial effect of lactobacilli and bifidobacteria in the prevention of antibiotic-associated or Clostridium difficile diarrhea.28 Administration of probiotics is suggested to prevent chemotherapy-associated diarrhea.29 Although probiotics may be effective in relieving global IBS symptoms, their efficacy for preventing diarrhea in patients with IBS is not clear. Data on the role of probiotics in the treatment of chronic diarrhea are currently lacking. The advantages of probiotics include mechanisms of action against pathogens and interaction with the host's natural defense systems. Disadvantages of probiotics include lack of evidence regarding the strain-specific effects, poor standardization for clinical trial designs, insufficient quality of some products, and variations in the microbial preparations.
ABSORBENT
Diosmectite is an absorbent consisting of a natural aluminum and magnesium silicate clay. It is known to improve stool consistency by absorption of toxins, bacteria, and viruses, reinforcement of the intestinal mucus barrier with the reduction of penetration of luminal antigens through the mucus layer, and reduction of inflammation. Previous studies have shown its efficacy in reducing the duration of acute diarrheal diseases.30 Diosmectite can be recommended for the treatment of both acute non-infectious and infectious diarrhea. Furthermore, it was reported to be effective in the treatment of IBS-D and chronic functional diarrhea.313233 Diosmectite can reduce the frequency of bowel movements and improve stool consistency in patients with chronic functional diarrhea. It is also indicated in the prevention of radiation-induced, chemotherapy-induced, and acquired immune deficiency syndrome-associated chronic diarrhea.343536
ANTISPASMODICS
Antispasmodics are a heterogeneous group of drugs that reduce smooth muscle contractility of the gut. Antispasmodics can be divided into two groups, based on the mechanism of action. Agents directly affecting intestinal smooth muscle by acting on calcium channels include alverine citrate, otilonium bromide, peppermint oil, pinaverium bromide, and mebeverine. Those with anticholinergic/antimuscarinic properties include butylscopolamine, hyoscine, cimetropium bromide, pirenzepine, dicyclomine, and prifinium bromide.3738 Anticholinergics reduce motility and secretion by blocking the binding of the neurotransmitter acetylcholine to its receptor. These agents can act on other body organs through muscarinic receptors, resulting in adverse effects such as dry mouth, tachycardia, and impaired vision. The agents acting on calcium channels slow colonic transit, improve stool consistency and frequency, and usually do not have the side effects of anticholinergics.
Alverine citrate is an antispasmodic agent modulating smooth muscle activity by inhibiting calcium uptake. In animal studies, alverine was reported to reduce the response of the gastrointestinal tract to mechanical and chemical stimuli.39 Reduced calcium influx may lead to decreased chemical sensitivity and smooth muscle relaxation.40 Alverine citrate can suppress both the frequency and amplitude of smooth muscle contractions of the gastrointestinal tract. Thus, it is used for the treatment of IBS, particularly in a combined form with simethicone. Simethicone potentiates the antinociceptive action of alverine. Combined treatment with alverine citrate and simethicone reduces abdominal pain and discomfort in patients with IBS.41
Mebeverine, a beta-phenylethylamine derivative of reserpine, blocks sodium channels and inhibits intracellular calcium accumulation.4243 It has no atropine-like side effects. Mebeverine was reported to reduce sigmoid colonic motility in hyperactive subjects, but its effect was less in hypoactive subjects.44 Previous studies demonstrated that mebeverine significantly reduced stool frequency and improved stool consistency.2045 In those studies, there were no significant side effects.
Otilonium bromide is a quaternary ammonium derivative with minimal systemic absorption from the gastrointestinal tract due to its structure. Thus, it has a selective antispasmodic action on the gastrointestinal tract, particularly on the colon.4647 It not only blocks L-type calcium-channels, but also binds to muscarinic receptors and tachykinin NK2 receptors.484950 Thus, otilonium bromide may have spasmolytic effects and antisecretory action. In addition, this agent decreases peripheral sensory afferent transmission to the central nervous system by antagonism of tachykinin NK2 receptors.4849 These mechanisms of action suggest that otilonium bromide could be effective in the treatment of IBS-D symptoms by reducing hypermotility and hypersensitivity. Studies have shown the efficacy of otilonium bromide in IBS patients.515253 Diarrheal symptoms and abdominal pain were significantly improved by otilonium bromide. Side effects of dry mouth, nausea, and dizziness can occur due to binding to muscarinic receptors. Pinaverium bromide is also a quaternary ammonium derivative with a low absorption rate from the gastrointestinal tract. Like otilonium bromide, it reduces abdominal pain and stool frequency.54
ANTIBIOTICS (RIFAXIMIN)
Most infectious diarrhea is acute and self-limited. Antibiotics are often recommended for febrile patients with moderate to severe diarrhea, patients with debilitating diseases, persistent diarrhea, traveler's diarrhea, and cases associated with parasites or more serious bacterial infections.
Rifaximin is a broad-spectrum oral antibiotic with limited systemic absorption that acts as an inhibitor of bacterial RNA synthesis. Poor absorption of rifaximin from the gastrointestinal tract enhances exposure to the intestine. It can be used to treat diarrhea associated with IBS and small intestinal bacterial overgrowth.5556 Rifaximin inhibits bacterial translocation across the epithelial lining of the gut. This agent impedes bacterial adherence to epithelial cells and down-regulates epithelial proinflammatory cytokine expression.57 These mechanisms of action appear to modulate the intestinal microbiota and gut immune signaling. The use of rifaximin in the treatment of IBS has been supported by the role of bacteria in the pathogenesis of IBS. Evidence suggests that treatment with rifaximin for 2 weeks improves global IBS symptoms such as bloating, abdominal pain, and loose or watery stools. It has been demonstrated to be particularly effective in the non-constipation subtypes of IBS.5859
ANTI-INFLAMMATORY AGENTS
Diarrhea is a common symptom of IBDs. The pathophysiology is complex, but impaired absorption of electrolytes and water by the inflamed bowel is believed to be the most important mechanism involved. Diarrhea in patients with IBDs may be caused by a variety of other conditions as well as inflammation itself. Thus, it is necessary to differentiate the underlying pathophysiologic mechanisms involved in diarrhea to determine appropriate therapy.
Microscopic colitis is a cause of chronic watery diarrhea. Although microscopic colitis has been increasingly diagnosed, it is still not a common condition. Its incidence is largely unknown. Microscopic colitis consists of two histopathologically different conditions: collagenous colitis and lymphocytic colitis. The three features of microscopic colitis include chronic watery non-bloody diarrhea, normal colonic mucosa on colonoscopy, and characteristic histopathology.6061 Patients usually present with a long history of watery non-bloody diarrhea. Although colonoscopy reveals normal mucosa, multiple colonic biopsies are required in order to make a diagnosis.
Nonspecific antidiarrheal agents such as loperamide are commonly used in the treatment of microscopic colitis. Mesalazine or 5-aminosalicylic acid (5-ASA) is an anti-inflammatory drug used to treat IBDs such as CD and UC. 5-ASA has frequently been used in patients with microscopic colitis. However, the efficacy of mesalazine in microscopic colitis is weak or remains uncertain. The evidence for the benefit of budesonide (9 mg/d) in the treatment of microscopic colitis is strongest, compared with other therapeutic agents. Budesonide is effective in both lymphocytic colitis and collagenous colitis.62 Budesonide was reported to be significantly superior to placebo and 5-ASA in collagenous colitis.63
CONCLUSIONS
Chronic diarrhea can be caused by multiple conditions. The underlying mechanisms are also diverse. A diagnostic approach for revealing underlying causes or mechanisms is required in order to administer specific therapies. When a specific treatment is not available, symptomatic management using pharmacologic agents for diarrhea should be initiated. Pharmacologic agents that can be used for the management of chronic diarrhea include loperamide, 5-HT3 receptor antagonists, diosmectite, cholestyramine, probiotics, antispasmodics, rifaximin, and anti-inflammatory agents (Table 1). Considering their mechanisms of action, these agents should be prescribed properly.
Notes
Financial support: None.
Conflict of interest: None.