Efficacy of serum leucine-rich alpha-2 glycoprotein in predicting findings of Crohn’s disease small bowel lesion in capsule endoscopy
Article information
Abstract
Background/Aims
Small bowel capsule endoscopy (SBCE) is an evaluation method for small bowel (SB) lesions in Crohn’s disease (CD). However, the relationship between SBCE findings and the serological biomarker leucine-rich alpha-2 glycoprotein (LRG) remains unclear. We aimed to establish appropriate cutoff values of LRG to predict the presence of SB lesions in CD through SBCE.
Methods
Patients with CD with SB lesions who had undergone SBCE and LRG measurements 1 month before and after the SBCE were included. The LRG values for ulcers ≥0.5 cm and other inflammatory lesions noted in SBCE were determined using the Youden Index, and the sensitivity and specificity were calculated. Additionally, the correlation between the SBCE scores (CD Activity in Capsule Endoscopy) and LRG values was evaluated.
Results
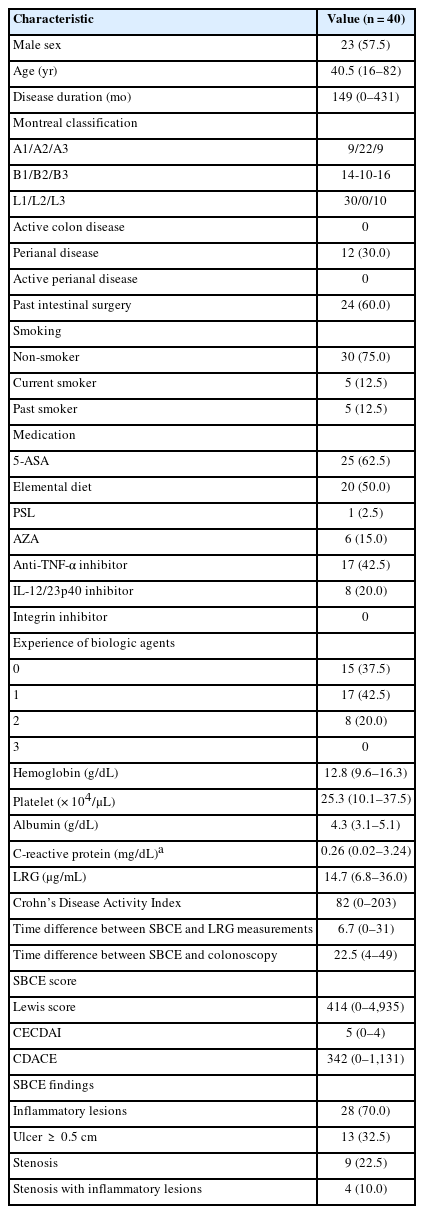
Forty patients without active colorectal lesions were included in the study. When the cutoff value of LRG for SB ulcers ≥ 0.5 cm was set at 14 μg/mL, the sensitivity was 92.3%, specificity was 81.5%, positive predictive value (PPV) was 70.6%, and negative predictive value (NPV) was 95.7%. In contrast, an LRG cutoff value of 12 μg/mL without inflammatory findings had a sensitivity of 91.7%, specificity of 82.1%, PPV of 68.8%, and NPV of 95.8%. CD Activity in Capsule Endoscopy correlated well with LRG values (Spearman’s rank correlation coefficient ρ = 0.681, P<0.001).
Conclusions
An LRG cutoff value of 14 μg/mL may be useful in predicting the presence of SB ulcers ≥ 0.5 cm, and an LRG cutoff value of 12 μg/mL may be useful in predicting the absence of SB inflammatory findings.
INTRODUCTION
In a recently reported cohort study of new-onset Crohn’s disease (CD) in Japanese patients, small bowel (SB) involvement was found in approximately 80% of patients with CD [1]. SB involvement is difficult to accurately assess with the Crohn’s Disease Activity Index (CDAI), which assesses the disease activity in patients with CD. Furthermore, it has been shown that C-reactive protein (CRP), which can be measured by blood sampling, may not reflect the disease activity of SB lesions in CD [2-5]. SB involvement in CD is an independent risk factor for relapse and surgery [2], and monitoring SB involvement in patients with CD who are in clinical remission is important.
Recently, leucine-rich alpha-2-glycoprotein (LRG) has attracted attention as a new biomarker for ulcerative colitis and CD [6,7]. LRG is a 50 kDa protein independent of IL-6 that is produced at inflammatory sites in the intestinal tract [6]. A previous study reported that LRG was useful in predicting the presence of SB ulcerative lesions in patients with CD who had a CDAI < 150 and CRP levels < 0.5 mg/dL, which is considered clinical remission [8]. This study aimed to establish a cutoff value of LRG for SB lesions based on SB capsule endoscopy (SBCE) findings.
METHODS
1. Study Design
This retrospective single-center study included patients with CD with Montreal classification L1 or L3 who visited our institute between July 2020 and January 2023. SBCE was performed to assess the activity of SB lesions in established CD with SB involvement. Inclusion criteria were the presence of intestinal patency by patency capsule and patients who had undergone SBCE. Exclusion criteria included active anorectal lesions, stoma, extraintestinal lesions, and other inflammatory diseases. Additionally, patients with active colon lesions detected in colonoscopy within 2 months of SBCE and those who had not undergone colonoscopy were excluded from the L3 CD cohort. Finally, 40 patients with CD were evaluated in this study (Fig. 1).
Study profile. Intestinal patency was evaluated by patency capsule in all cases. CD, Crohn’s disease; SB, small bowel; SBCE, SB capsule endoscopy; LRG, leucine-rich alpha-2 glycoprotein.
SB lesions were evaluated by SBCE, which allows imaging of the entire SB. Because the presence of SB ulcerative lesions > 5 mm affects prognosis [2], SBCE findings were defined as follows: mucosal breaks with white or yellow bases with ≥ 0.5 cm mucosal defects were defined as ulcers, and mucosal breaks with white or yellow bases with < 0.5 cm mucosal defects were defined as erosions. The diameter of a typical small intestine was assumed to be 2.5 cm, and the circumference of 1/4 of the small intestine was calculated as 2 cm. Based on these, the size of the ulcer was estimated [8]. Erythema and intestinal edema were defined as other inflammatory lesions (Supplementary Fig. 1). Stenosis was defined as a white ring that occurs when the SBCE passes or is difficult to pass. SBCE scores were calculated using the Lewis score (LS) [9], the Capsule Endoscopic Crohn’s Disease Activity Index (CECDAI) [10], and Crohn’s Disease Activity in Capsule Endoscopy (CDACE) score [11]. Serological markers such as LRG and the CDAI were measured within 1 month of SBCE. SBCE evaluation was performed by a single expert with experience of over 1,500 SBCE readings. LRG and other blood collection data were verified after the SBCE evaluation.
The primary endpoints were the presence of SB ulcerative lesions and erosions or other inflammatory findings. The measured LRG values were used as the cutoff values from the receiver operating characteristic curve established using the Youden Index, and the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), negative likelihood ratio (–LR) and accuracy were calculated. Regarding the presence of SB ulcerative lesions, cutoff values were also calculated for CDAI and CRP values and compared. The correlation between LRG values and SBCE scores was evaluated as a secondary endpoint.
2. Ethical Considerations
The study protocol was approved by the Human Ethics Review Committee of our university (October 2, 2023; approval no. 2022-0125). Informed consent was obtained from all patients using an opt-out method because of the retrospective design of the study.
3. Statistical Analysis
Numerical data are expressed as the average and range. Wilcoxon, Fisher exact, and Kruskal-Wallis tests were used in the univariate analysis of background factors. Statistical significance was set at a P-value < 0.05. Regarding the presence of ulcers (≥ 0.5 cm) and all inflammatory lesions (ulcer [ ≥ 0.5 cm], erosion [ < 0.5 cm], and redness and edema), the cutoff value for each score was calculated from the receiver operating characteristic curve, and the sensitivity, specificity, PPV, NPV, +LR, –LR, and accuracy were computed. The reliability of each value was assessed by calculating 95% confidence intervals (CIs). Spearman’s rank correlation coefficient was used to analyze the correlations of the LRG value, CRP value, and CDAI with the CDACE, LS, and CECDAI. JMP statistical analysis software (version 16; SAS Institute Inc., Cary, NC, USA) was used for all analyses.
RESULTS
The background information of the 40 patients is shown in Table 1. The mean age was 40.5 years (range, 16–82 years), and 23 patients (57.5%) were men. Based on the Montreal classification, 14 cases (35%) were inflammatory (B1), 10 cases (25%) were stenotic (B2), 16 cases (40%) were perforated (B3), 30 cases (75%) were L1, and 10 cases (25%) were L3. The mean CDAI was 82 (range, 0–203) at the time of SBCE, the mean CRP level was 0.26 mg/dL (range, 0.02–3.24 mg/dL), and the mean LRG value was 14.7 μg/mL (range, 6.8–36.0 μg/mL). Based on SBCE findings, 13 patients (32.5%) had SB ulcerative lesions ≥ 0.5 cm in diameter; 28 patients (70%) had inflammatory findings such as edema, erythema, erosions, and ulcers ≥ 0.5 cm; and 9 patients (22.5%) had stenosis that allowed the passage of a patency capsule. SBCE retention did not occur in any case. The mean SBCE scores were as follows: LS, 414 (0–4,935); CECDAI, 5 (0–14); and CDACE, 342 (0–1,131).
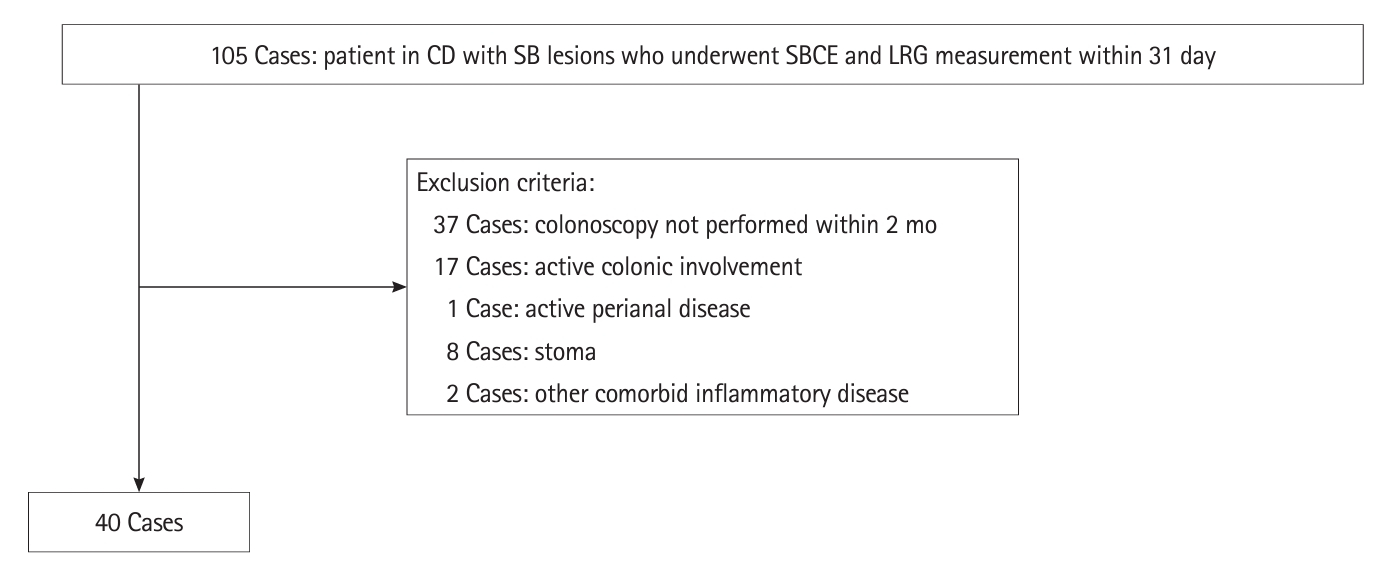
The area under the curve (AUC) for the presence of SB ulcers ≥ 0.5 cm was the highest for an LRG value of 14 μg/mL (AUC, 0.92; 95% CI, 0.84–0.10) (Fig. 2). When the cutoff value of LRG was 14 μg/mL, the presence of SB ulcer ≥ 0.5 cm could be discriminated with a sensitivity of 92.3%, specificity of 81.5%, PPV of 70.6%, NPV of 95.7%, +LR of 4.985, –LR of 0.094, and accuracy of 85.0% (Table 2). The AUCs of the existing serological marker and clinical activity (CRP 0.14 mg/dL and CDAI 85) were maximum; however, both were smaller than the AUC of LRG (CRP 0.70 mg/dL and CDAI 0.68) (Fig. 2).
Receiver operating characteristic curve of CDAI, CRP, and LRG for small bowel ulcer (≥0.5 cm). The highest AUC value was 0.92 at an LRG value of 14 μg/mL. CDAI, Crohn’s Disease Activity Index; CRP, C-reactive protein; LRG, leucine-rich alpha-2 glycoprotein; AUC, area under the curve.
The AUC for the absence of inflammatory findings such as edema, erythema, erosions, and ulcers ≥ 0.5 cm was the highest for an LRG value of 12 μg/mL (AUC, 0.87; 95% CI, 0.77–0.98) (Fig. 3). When the LRG cutoff value was set as 12 μg/mL, the absence of inflammatory findings could be discriminated with a sensitivity of 91.7%, specificity of 82.1%, PPV of 68.8%, NPV of 95.8%, +LR of 5.133, –LR of 0.101, and accuracy of 85.0%, indicating that there were no inflammatory findings based on SBCE (Table 2).
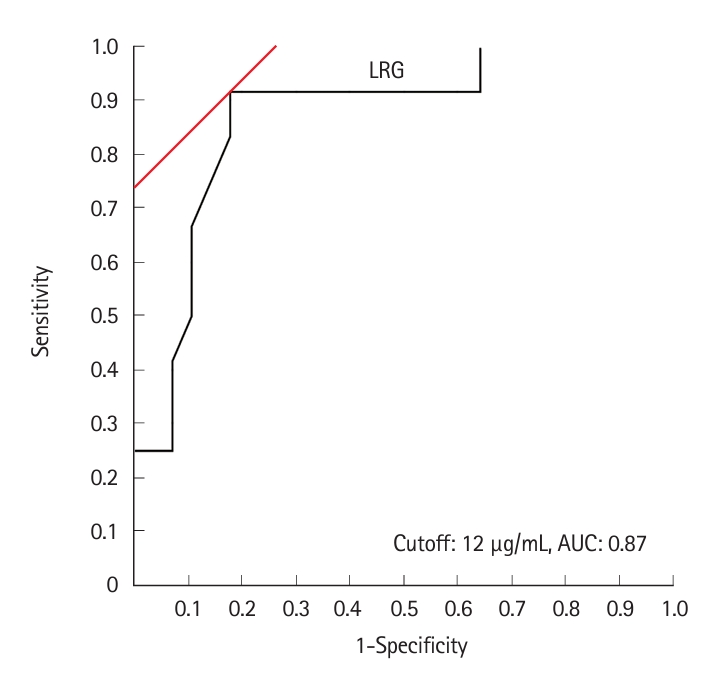
Receiver operating characteristic curve of leucine-rich alpha-2 glycoprotein (LRG) levels for the absence of small bowel inflammatory findings in patients with Crohn’s disease. The maximum area under the curve (AUC) value was 0.87 at an LRG value of 12 μg/mL.
All 40 cases were grouped based on the following LRG values: < 12 μg/mL (n = 7), 12–14 μg/mL (n = 16), and > 14 μg/mL (n = 17). When comparing the LRG > 14 μg/mL and ≤ 14 μg/mL groups, ulcers ≥ 0.5 cm were more common in the LRG value > 14 μg/mL group, and a significant difference was observed (P<0.001). In the LRG > 14 μg/mL and 12–14 μg/mL groups, ulcers ≥ 0.5 cm were more common in the LRG > 14 μg/mL group (P=0.023).
SBCE scores correlated well with LRG values: LS, ρ= 0.612 (P<0.001); CECDAI, ρ= 0.661 (P<0.001); CDACE, ρ= 0.681 (P<0.001). CDACE showed the highest correlation (spearman rank correlation coefficient), while no correlation was observed between the SBCE scores and CDAI (Table 3, Fig. 4, Supplementary Fig. 2).
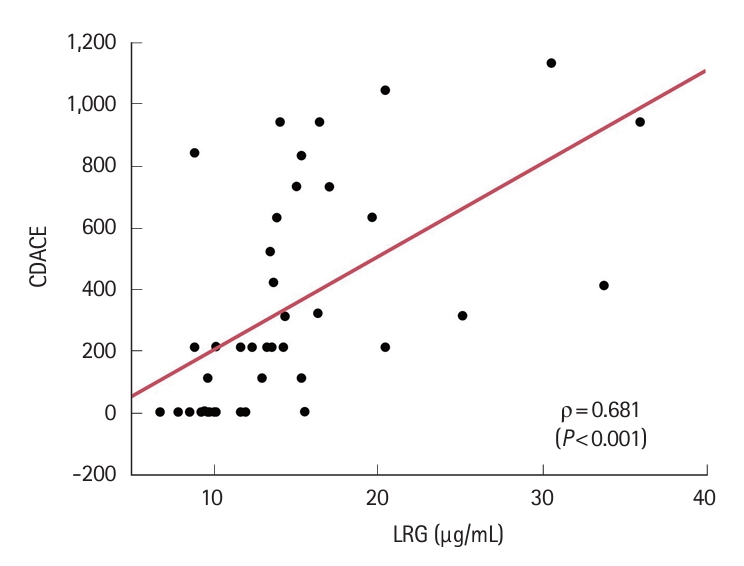
Correlation between CDACE and LRG (Spearman's rank correlation coefficient). CDACE, Crohn’s Disease Activity in Capsule Endoscopy; LRG, leucine-rich alpha-2 glycoprotein.
In addition, LRG values based on the different localizations of small intestinal inflammation were compared. In patients with L3 CD, the presence of inflammation of the terminal ileum was determined based on the results of the most recent colonoscopy and SBCE findings. Patients with L1 CD did not have a recent colonoscopy; therefore, the presence of inflammation of the terminal ileum was determined based on SBCE findings. Fifteen patients had inflammation of the terminal ileum. Of these, 6 had inflammation only in the terminal ileum. Except for the 12 patients with no inflammatory findings on SBCE, there was no significant difference in LRG values between the group with inflammation in the small intestine other than the terminal ileum (n = 13), the group with inflammation only in the terminal ileum (n = 6), and the group with inflammation in the small intestine including the terminal ileum (n = 9). However, the group with inflammation in the small intestine, including the terminal ileum, tended to have higher LRG values (14.2 [8.9–25.2] vs. 17.6 [10.2–33.8] vs. 19.8 [8.9–36.0], P=0.120).
There was a significant difference in LRG values between patients with and without SB ulcerative lesions (17.1 [14.8–27.9] μg/mL vs. 11.7 [9.5–13.7] μg/mL; P<0.001). There were also significant differences in LRG values between patients with and without inflammatory findings such as edema, erythema, erosions, and ulcerations ≥ 0.5 cm (14.4 [13.1–19.1] μg/mL vs. 9.8 [8.8–11.3] μg/mL; P=< 0.001). In contrast, there was a significant difference in CRP levels between patients with and without SB ulcerative lesions (0.18 [0.07–0.54] mg/dL vs. 0.07 (0.04–0.11) mg/dL; P=0.040), while there was no significant difference in CRP levels between patients with and without inflammatory findings (0.09 [0.06–0.23] mg/dL vs. 0.05 [0.03–0.17] mg/dL; P=0.071).
DISCUSSION
It has been reported that SB lesions in CD are not easily reflected in clinical symptoms and cannot be determined by conventional markers such as CRP values [2-5]. In contrast, SB involvement is an independent risk factor for relapse and surgery, making monitoring of SB involvement clinically relevant [2]. The evaluation of disease activity in patients with CD using LRG levels has been recently reported in a meta-analysis of 9 studies [12], which showed a sensitivity and specificity of 77.0% (95% confidence interval [CI], 67.8% to 84.2%) and 81.1% (95% CI, 72.6% to 87.4%), respectively [12]. In addition, the usefulness of LRG in detecting endoscopic disease activity has been reported to be similar to that of fecal calprotectin levels in patients with CD [13,14].
There are several reports regarding the prediction of SB lesions using balloon-assisted enteroscopy (BAE) [15-17]. The cutoff value of LRG was 13.4 μg/mL for ulcerative lesions ≥ 0.5 cm, with a detection sensitivity of 79%, specificity of 82%, PPV of 93%, and NPV of 58% [15]. In contrast, when the LRG cutoff value for endoscopic remission, in which erosions and ulcers disappeared, was set at 8.9 μg/mL, the detection sensitivity was 93.3%, specificity was 83.3%, PPV was 96.6%, NPV was 71.4%, accuracy was 91.7%, and the AUC for predicting endoscopic remission was 0.904 [17]. This suggests that the cutoff value of LRG in BAE may differ depending on the target. In a study of patients with CDAI < 150 and CRP < 3 mg/L, Asonuma et al. [18] reported that an LRG value of 16 μg/mL had the highest PPV for the presence of ulcerative lesions, and an LRG value of 9 μg/mL had the highest NPV. However, they used a variety of modalities, such as SBCE, BAE, magnetic resonance enterography (MRE), and intestinal ultrasonography, which may account for the observed difference.
A study using MRE as the single detection modality reported a sensitivity of 67% and specificity of 90% for an inflammation-specific simplified MRE index of activity (sMaRIA) score of ≥ 4 when the LRG cutoff value was ≥ 14 μg/mL [19]. The sMaRIA score ≥ 4 generally indicated the presence of ulcers ≥ 0.5 cm; the LRG values observed were similar to those in this study.
This study compared LRG values with the entire SB lesions using SBCE as a single modality without including the colorectal lesions; an LRG cutoff value of 14 μg/mL indicated the presence of a ≥ 0.5 cm SB ulcer, while an LRG cutoff value of 12 μg/mL was a possible predictor of no inflammatory findings. These cutoff values had high sensitivity and specificity but low PPV and high NPV. Conversely, a significant difference was observed between the LRG > 14 μg/mL and 12–14 μg/mL groups, with more ulcers > 0.5 cm in the LRG > 14 μg/mL group. Thus, the practical interpretation of LRG values in SB lesion detection by SBCE may be as follows: LRG values of 12–14 μg/mL may detect edema, erythema, and erosions rather than ulcers ≥ 0.5 cm.
A positive correlation between LRG levels and SBCE scores was also observed in this study. Since some reports [19-21] have shown that higher LRG levels are more strongly associated with CD-related hospitalization, surgery, and clinical recurrence, an LRG value ≥ 14 μg/mL may be an indicator for SBCE examination. In contrast, an LRG value < 14 μg/mL indicates small lesions that SBCE can detect; however, there is no clear evidence that their presence affects prognosis. Furthermore, an LRG value ≤ 12 μg/mL may be an acceptable indicator to skip evaluation by SBCE according to the T2T strategy [22] since small lesions may also be absent (Fig. 5).
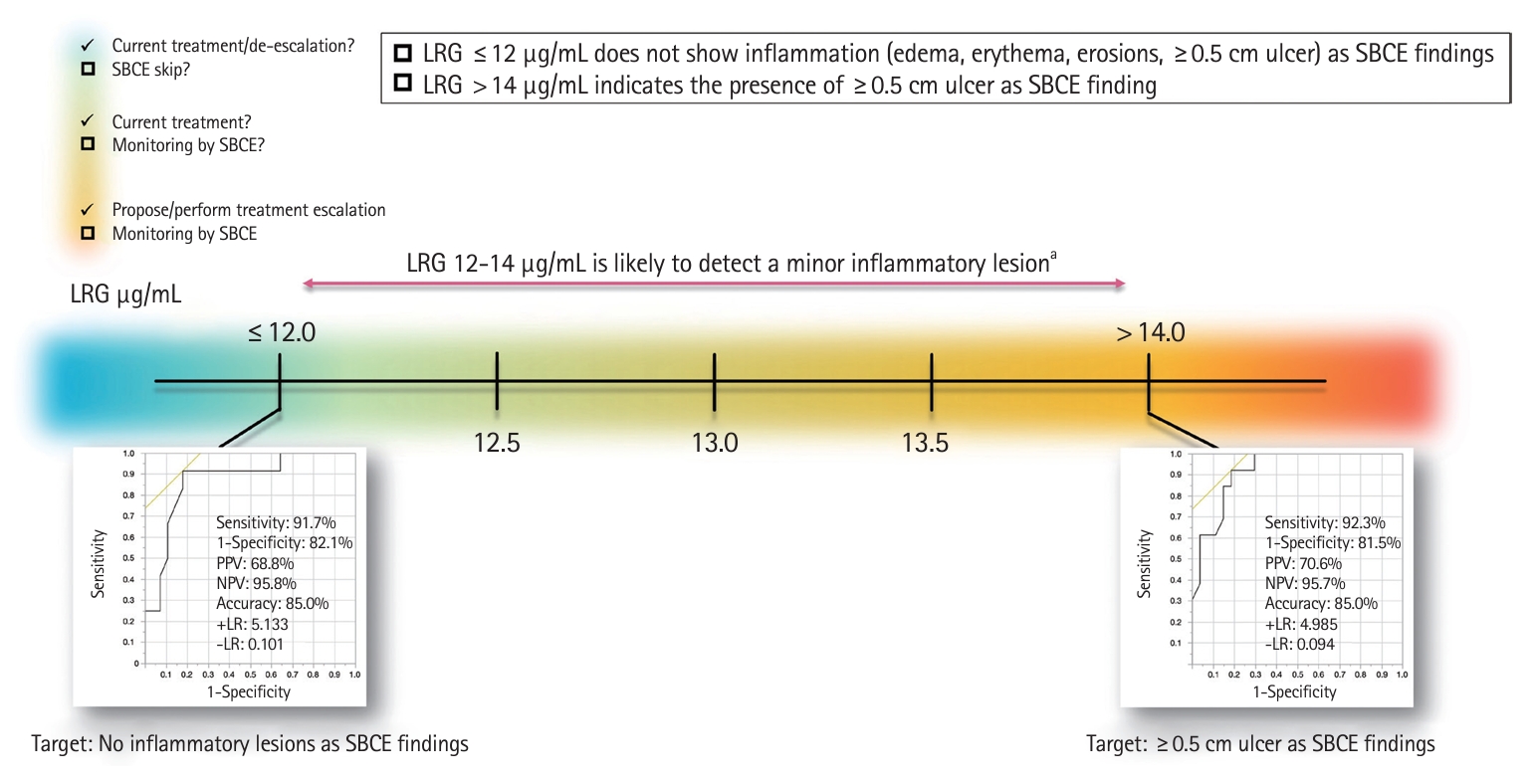
Prediction of the presence of SB lesions based on LRG values and proposed interpretation. aMinor inflammatory lesions: edema, erythema, erosions (<0.5 cm mucosal lesions). SB, small bowel; SBCE, SB capsule endoscopy; LRG, leucine-rich alpha-2 glycoprotein; PPV, positive predictive value; NPV, negative predictive value; +LR, positive likelihood ratio; –LR, negative likelihood ratio.
A study previously reported that LRG was useful in predicting the presence of ≥ 0.5 cm SB ulcerative lesions in patients with CD with CDAI < 150 and CRP < 0.5 mg/dL, which is considered clinical remission [8]; however, colonic lesion activity was not assessed in previous studies. Herein, patients with Montreal Classification L3 were also included, but colonoscopy was performed within 2 months to confirm the absence of active colorectal lesions. Therefore, in our study, colorectal lesions did not affect LRG value which may have revealed real SB lesions.
In this study, there was no significant difference in LRG values between the groups with small intestinal inflammation outside of the terminal ileum, the group with inflammation only in the terminal ileum, and the group with inflammation in the small intestine, including the terminal ileum. However, the group with inflammation in the small intestine, including the terminal ileum, tended to have higher LRG levels (14.2 [8.9–25.2] vs. 17.6 [10.2–33.8] vs. 19.8 [8.9–36.0], P=0.119). Although the terminal ileum lesion is considered important, LRG values may be higher in patients with inflammation involving the entire SB. If LRG values are high despite a paucity of terminal ileal lesions, there may be ulcerative lesions ≥ 0.5 cm and/or extensive inflammatory findings in the deep small intestine that cannot be assessed by ileocolonoscopy; therefore, it may be advisable to evaluate the entire small intestine.
This study has some limitations. The patients included in the study underwent patency evaluations using patency capsules; LRG cutoff values may differ in patients with SB stenoses that do not allow passage of the patency capsule. The SBCE evaluation was performed by a single expert. This lack of SBCE evaluation by more than one person is a limitation of the quality of the study. Although we defined an ulcerative lesion as a mucosal defect of ≥ 0.5 cm, size is not considered in the definition of an ulcer lesion in SBCE [23,24]. Moreover, since this is a retrospective study of a small number of patients conducted at a single institution, caution should be exercised in interpreting the results. Therefore, validation in a multicenter study with a large number of patients is desirable.
In conclusion, our results suggest that an LRG value ≥ 14 μg/mL may be useful in predicting the presence of SB ulcers ≥ 0.5 cm and an LRG value ≤ 12 μg/mL may be useful in predicting the absence of SB inflammatory findings in patients with CD with SB lesions.
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Author Contributions
Conceptualization: Omori T. Date curation: Murasugi S, Koroku M, Ito A, Yonezawa M, Omori T. Formal analysis: Omori T. Writing - original draft: Omori T. Writing – review & editing: Omori T, Nakamura S, Tokushige K. Approval of final manuscript: all authors.
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Fig. 1.
Erythema (A) and intestinal edema (B).
Supplementary Fig. 2.
Correlation between LS, CECDAI, and LRG (Spearman’s rank correlation coefficient). LS, Lewis score; CECDAI, Capsule Endoscopy Crohn’s Disease Activity Index; LRG, leucine-rich alpha-2 glycoprotein.