Accuracy of three different fecal calprotectin tests in the diagnosis of inflammatory bowel disease
Article information
Abstract
Background/Aims
Several studies have found that the measurement of fecal calprotectin is useful for the early diagnosis of inflammatory bowel disease (IBD). We compared the effectiveness of three different fecal calprotectin kits for initial diagnosis in patients with suspected IBD.
Methods
We enrolled 31 patients with IBD (18 Crohn's disease [CD], 11 ulcerative colitis [UC], and two intestinal Behçet's disease), five with irritable bowel syndrome (IBS), and five with other colitis (four infectious colitis and one intestinal tuberculosis). Diagnosis was based on clinical, laboratory, and endoscopic examinations. Fecal samples were obtained at the first diagnosis and calprotectin levels were measured using three different kits (Quantum Blue® Calprotectin, EliA™ Calprotectin, and RIDASCREEN® Calprotectin).
Results
The overall accuracy for differentiating IBD from IBS or other colitis was 94% and 91%, respectively, for Quantum Blue® (cutoff, 50 µg/g); 92% and 89%, respectively, for EliA™ (cutoff, 50 µg/g); and 82% and 76%, respectively, for RIDASCREEN® (cutoff, 50 µg/g). In patients with CD, the results of Quantum Blue® Calprotectin and EliA™ Calprotectin correlated significantly with levels of the Crohn's disease activity index (Spearman's rank correlation coefficient, r=0.66 and r=0.49, respectively). In patients with UC, the results of EliA™ Calprotectin correlated significantly with the Mayo score (r=0.70).
Conclusions
Fecal calprotectin measurement is useful for the identification of IBD. The overall accuracies of the three fecal calprotectin kits are comparable.
INTRODUCTION
Fecal calprotectin has been proposed as a candidate diagnostic marker for discrimination between IBD and IBS. Calprotectin is a heterodimer of the calcium-binding proteins S100A8 and S100A9 and is present mainly in neutrophils.1 Neutrophils migrate into the bowel lumen as a result of an inflammatory process, and the level of fecal calprotectin strongly correlates with the degree of intestinal inflammation.2 Moreover, calprotectin concentration distinguishes IBD from normal findings or functional disorders such as IBS.3 Several studies have shown that the calprotectin level can be used to identify IBD,4567 to assess the response to IBD therapy,7891011121314 and to predict an IBD relapse.715161718 Furthermore, fecal calprotectin analysis is noninvasive and easily accessible in comparison to colonoscopy with a biopsy, which is the gold standard for assessment of gastrointestinal inflammation.1920
Since identification of fecal calprotectin as a potential biomarker, many types of calprotectin test kits have been developed. ELISAs have been typically used to quantify fecal calprotectin. Recently, quantitative immunochromatographic point-of-care tests or immunoassays were developed as a reliable alternative to the more time-consuming ELISA methods.2122 Nonetheless, these calprotectin test kits vary in characteristics, and few data are available regarding clinical comparison. A comparison of different calprotectin test kits may help clinicians in choosing the appropriate assay.
We designed this study to evaluate the initial diagnostic ability of three fecal calprotectin kits−Quantum Blue® Calprotectin, EliA™ Calprotectin, and RIDASCREEN® Calprotectin−in different bowel diseases. Furthermore, we tested whether the results produced by these fecal calprotectin kits correlate with disease activity and location of CD or UC.
METHODS
1. Subjects
We screened 52 patients who visited Severance Hospital from July to December 2014 for a workup because of abdominal pain, changes in bowel habits, and/or anorectal bleeding. We excluded patients who met any of the following criteria: incomplete colonoscopy, inability to collect fecal samples, colorectal cancer, history of bowel resection, an uncertain diagnosis after colonoscopy, or regular intake of aspirin and/or NSAIDs. Of a total of 52 screened patients, 11 were excluded: two for a lack of fecal samples, one for colorectal cancer, and eight for unclear diagnoses (Fig. 1). Finally, 41 patients were enrolled in this study.
The patients were classified into three groups: IBD (CD, UC, and intestinal Behçet's disease), IBS, and "other colitis." The latter group was defined as patients with a bowel disease other than IBD and IBS and included patients with infectious colitis and intestinal tuberculosis. The diagnosis of a bowel disease was established on the basis of a clinical history, laboratory results, endoscopic findings, pathology, and radiologic analyses according to the diagnostic criteria. We also assessed disease severity using CDAI in patients with CD and the Mayo score in patients with UC. CRP was quantified by an immunoturbidimetric method and the cutoff was 8 mg/L. All the enrolled patients underwent complete colonoscopy. The data were collected in a prospective manner and analyzed retrospectively.
The study protocol adheres to the 1975 Declaration of Helsinki guidelines and was approved by the Institutional Review Board of Yonsei University College of Medicine (approval number: 4-2015-1117). Each participant or legal guardian provided written informed consent for diagnosis and treatment.
2. Fecal Calprotectin
Fecal samples were obtained at the first diagnosis and fecal calprotectin levels were measured with three kits: Quantum Blue® Calprotectin, EliA™ Calprotectin, and RIDASCREEN® Calprotectin. The laboratory technician conducting the tests was blinded to the patient's clinical information. All the tests were performed according to the manufacturers' instructions.
The Quantum Blue® Calprotectin kit (Bühlmann Laboratories, Basel, Switzerland) was provided by WOONGBEE MeDiTech (Seongnam, Korea). Fecal samples were diluted 1:50, and the cutoff was 50 µg/g. EliA™ Calprotectin (Phadia AB, Uppsala, Sweden) was provided by Phadia Korea (Seoul, Korea). Fecal samples were diluted 1:100, and the cutoff was 50 µg/g. The RIDASCREEN® Calprotectin kit (R-Biopharm AG, Darmstadt, Germany) was provided by Asan Pharmaceuticals (Seoul, Korea). Fecal samples were diluted 1:50, and the cutoff was 50 µg/g.
3. Statistical Analysis
The comparison among the three different calprotectin test kits was conducted using Passing and Bablok regression and Spearman's rank correlation. Clinical and laboratory data on the patients were presented as mean±SD, range, or n (%), when appropriate. The Mann-Whitney test was used to compare the results of quantitative assays for fecal calprotectin and CRP between each disease group and to evaluate a possible correlation between fecal calprotectin concentration and disease location in patients with IBD (a P-value <0.017 was considered statistically significant according to Bonferroni's correction). The test performance of the three different calprotectin kits and a CRP assay was evaluated by means of sensitivity, specificity, positive and negative predictive values, and overall accuracy. The association between disease activity and fecal calprotectin was evaluated by means of Spearman's rank correlation coefficient (r) for nonparametric correlations. A P-value <0.05 was assumed to denote statistical significance. All statistical analyses were performed in the SPSS software, version 12.0 (SPSS Inc., Chicago, IL, USA), except for the Passing and Bablok regression, which was analyzed in the MedCalc software, version 16.2.1 (MedCalc, Ostend, Belgium). Vertical scatter plots were constructed using GraphPad Prism, version 6 (Graphpad Software Inc., LaJolla, CA, USA).
RESULTS
1. Patients' Characteristics
A total of 41 patients were analyzed in this study. Clinical characteristics of these patients are shown in Table 1. Twelve of the patients with CD had ileocolonic involvement, one had colonic involvement, and five had ileal involvement. Of the patients with UC, five had proctitis, and six had pancolitis.
Mean disease activity of CD patients was 199.3 points (range, 133−393 points). There were 14 patients (78%) with CDAI >150. Mean disease activity among UC patients was 6.4 (range, 3−10). Five patients (45%) had Mayo scores of 3 to 5 (mildly active disease), and six patients (55%) had scores of 6 to 10 (moderately active disease).
The "other colitis" group consisted of four patients with infectious colitis and one patient with intestinal tuberculosis. Endoscopic findings revealed that the patients with infectious colitis had mucosal redness, erosions, and/or ulcers, and the patient with intestinal tuberculosis had circular ulcers with scars.
2. Comparison of the Methods
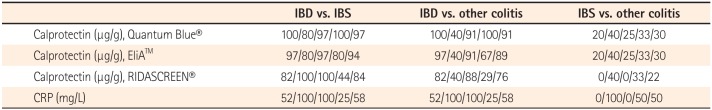
The correlation analysis was performed among Quantum Blue® (as a standard value), EliA™, and RIDASCREEN® (Fig. 2). Between Quantum Blue® and EliA™, the intercept of the regression line according to the Passing and Bablok regression was 7.19 (95% CI, 23.82−39.23) and no significant deviation from 0 was present. The slope was 0.39 (95% CI, 0.32−0.47) and a significant deviation from 1 was present. The intercept and slope were 54.70 (95% CI, 1.20−107.67) and 0.09 (95% CI, 0.07−0.17) between Quantum Blue® and RIDASCREEN®, and a significant deviation from 0 and 1 was present. Spearman's rank correlation coefficient was 0.90 (P<0.0001; 95% CI, 0.82−0.95) between Quantum Blue® and EliA™ and 0.72 (P<0.0001; 95% CI, 0.52−0.85) between Quantum Blue® and RIDASCREEN®. Although the three assays correlated significantly, the slopes and intercepts differed in pairwise comparisons.
The comparison of methods using Spearman's rank correlation. (A) The correlation and trend line between Quantum Blue® and EliA™ assays (n=39, r=0.90, P<0.0001; 95% CI, 0.82−0.95). (B) The correlation and trend line between Quantum Blue® and RIDASCREEN® assays (n=36, r=0.72, P<0.0001; 95% CI, 0.52−0.85).
3. Test Characteristics of Fecal Calprotectin and CRP
The results of quantitative assays for fecal calprotectin are presented in Fig. 3. The mean calprotectin level according to Quantum Blue® was 3,384±3,450 µg/g (range, 218−9,960 µg/g) in IBD, 120±190 µg/g (range, 30−459 µg/g) in IBS, and 227±184 µg/g (range, 30−394 µg/g) in "other colitis." According to EliA™, the mean calprotectin level was 1,476±1,127 µg/g (range, 47−3,000 µg/g) in IBD, 54±74 µg/g (range, 15−187 µg/g) in IBS, and 88±58 µg/g (range, 15−156 µg/g) in "other colitis." According to RIDASCREEN®, the mean calprotectin level was 467±360 µg/g (range, 0−800 µg/g) in IBD, 11±13 µg/g (range, 0−27 µg/g) in IBS, and 73±48 µg/g (range, 0−114 µg/g) in "other colitis." The mean CRP concentration was 17.0±21.8 mg/L (range, 0.3−72.7 mg/L) in IBD, 0.9±0.8 mg/L (range, 0.4−2.3 mg/L) in IBS, and 0.9±0.8 mg/L (range, 0.4−2.3 mg/L) in "other colitis." Fecal calprotectin levels according to the three kits and CRP levels were significantly higher in patients with IBD than in patients with IBS (P<0.017, Bonferroni's correction). Fecal calprotectin concentrations determined by the three different kits and CRP levels were not different between IBS and "other colitis" (P>0.017 according to Bonferroni's correction).

Fecal calprotectin measured by the three fecal calprotectin kits in three groups of patients. Fecal calprotectin according to the three different kits was significantly elevated in IBD compared to IBS. Fecal calprotectin determined by the three kits was not different between IBS and the "other colitis" group (see the METHODS section for the definition).
4. Test Performance of the Three Calprotectin Kits and CRP Assay
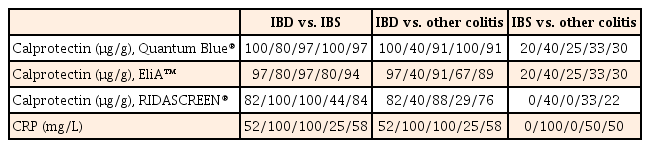
The test performance (sensitivity/specificity/positive predictive values/negative predictive values) and overall accuracy in distinguishing between IBD and IBS or "other colitis" is summarized in Table 2. For differentiation of IBD from IBS, the overall accuracy was 97%/94%/84%/58% for Quantum Blue®, EliA™, RIDASCREEN®, and CRP, respectively. For discrimination of IBD and "other colitis," the overall accuracy was 91%/89%/76%/58% for Quantum Blue®, EliA™, RIDASCREEN®, and CRP, respectively. Neither fecal calprotectin levels nor the CRP levels were helpful for differentiation of IBS and "other colitis" (accuracy, 22%−50%).
5. Correlation of Fecal Calprotectin Levels with Disease Activity among Patients with IBD
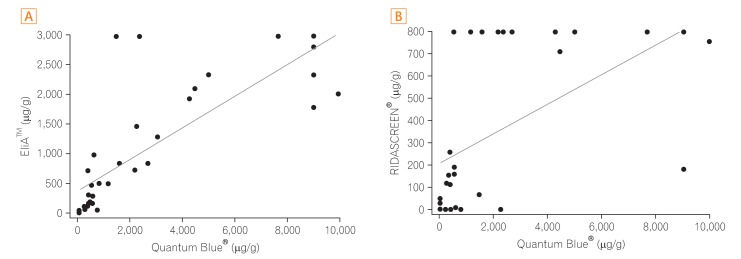
Data on the correlation between fecal calprotectin levels determined by the three kits and disease activity are depicted in Fig. 4. In patients with CD, Quantum Blue® Calprotectin and EliA™ Calprotectin correlated significantly with CDAI (P=0.004 and P=0.041, respectively; r=0.66 and r=0.49, respectively). The RIDASCREEN® calprotectin level did not correlate statistically significantly with CDAI. In patients with UC, the mean time interval between fecal sampling and colonoscopy was 12.7 days (median, 10 days; range, 0−32 days). EliA™ Calprotectin correlated significantly with the Mayo score (P=0.017, r=0.70), whereas Quantum Blue® Calprotectin and RIDASCREEN® calprotectin results did not correlate with the Mayo score.

(A-C) Correlation of the disease activity index with fecal calprotectin levels in IBD. In patients with CD, Quantum Blue® Calprotectin and EliA™ Calprotectin correlated significantly with the levels of CDAI. In patients with UC, EliA™ Calprotectin correlated significantly with the Mayo score. r, Spearman's rank correlation coefficient.
6. Correlation of Fecal Calprotectin Levels with the Disease Location in Patients with IBD
We also explored the relation between fecal calprotectin levels and the disease location in patients with IBD (Fig. 5). CD was located in the ileum in five patients, in the colon in one patient, and in the ileocolon in 12 patients. The mean calprotectin level according to Quantum Blue® was 1,156±975 µg/g (range, 256−2,250 µg/g) in the ileum, 4,450 µg/g in the colon, and 5,769±3,192 µg/g (range, 1,555−9,960 µg/g) in the ileocolon. According to EliA™, the mean calprotectin concentration was 569±576 µg/g (range, 53−1,471 µg/g) in the ileum, 2,103 µg/g in the colon, and 2,202±834 µg/g (range, 833−3,000 µg/g) in the ileocolon. According to RIDASCREEN®, the mean calprotectin concentration was 214±335 µg/g (range, 0−800 µg/g) in the ileum, 709 µg/g in the colon, and 796±13 µg/g (range, 758−800 µg/g) in the ileocolon. According to all three fecal calprotectin kits, fecal calprotectin levels were significantly higher in CD patients with ileocolonic involvement than in CD patients with ileal involvement (all P=0.004). CDAI did not differ statistically significantly when stratified by disease location (all P>0.05). UC was identified as a type of proctitis in five patients, and pancolitis in six patients. The mean calprotectin level according to Quantum Blue® was 2,166±3,817 µg/g (range, 218−8,990 µg/g) in proctitis and 2,353±3,742 µg/g (range, 376−9,000 µg/g) in pancolitis. According to EliA™, the mean calprotectin concentration was 947±1,199 µg/g (range, 116−3,000 µg/g) in proctitis and 1,515±1,273 µg/g (range, 283−3,000 µg/g) in pancolitis. According to RIDASCREEN®, the mean calprotectin concentration was 235±328 µg/g (range, 0−800 µg/g) in proctitis and 226±295 µg/g (range, 0−800 µg/g) in pancolitis. For all three kits, fecal calprotectin levels of patients with UC did not differ by disease location. The Mayo score was higher in pancolitis UC than in proctitis UC (7.7±2.1 vs. 4.8±1.5, respectively, P=0.033). In two patients with intestinal Behçet's disease, one patient's lesion was located in the ileocecal valve, and the other patient's lesions were located in the ileocecal valve, terminal ileum, and colon. Calprotectin levels of the former and latter were 736 µg/g and 1,128 µg/g according to Quantum Blue®, 47 µg/g and 500 µg/g according to EliA™, and untestable (due to a lack of fecal samples) and above 800 µg/g according to RIDASCREEN ®, respectively.
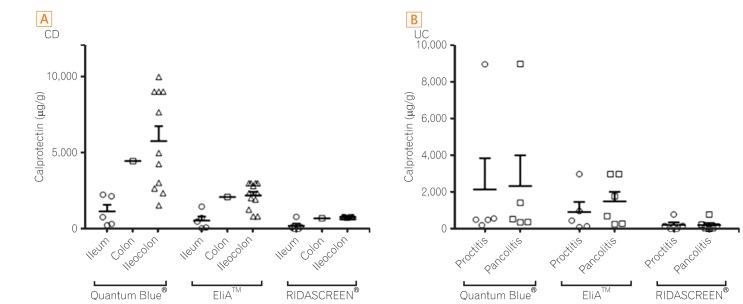
Correlation of fecal calprotectin levels with the disease location in IBD. Solid lines indicate the mean of each group and error bars represent one standard error. (A) CD. According to all three kits, fecal calprotectin levels were significantly higher for ileocolonic involvement than for ileal involvement (all P-values, 0.004). (B) UC. For all three kits, fecal calprotectin levels did not differ by disease location.
DISCUSSION
Calprotectin, which was first described in 1980, has been proposed as a possible diagnostic marker of IBD.23 Conventional testing for calprotectin involves a laboratory ELISA, but the ELISA method is time-consuming, has a long turnaround time, and requires expertise. Recently, quantitative immunochromatographic point-of-care tests or quantitative enzyme immunoassays have become available as alternatives to the time-consuming ELISA. These tests can be performed in the clinic within 15 minutes. Only a small amount of stool is required, and the testing can be performed on stool samples kept for up to 1 week at room temperature because fecal calprotectin is resistant to bacterial degradation. Therefore, patients can bring a sample from home and receive a result on fecal calprotectin in a single visit.
In 2012, Coorevits et al.21 reported a comparison of a newly developed quantitative immunochromatographic point-of-care test (Quantum Blue®) with an established ELISA test in terms of distinguishing organic from functional bowel diseases. The determination coefficient (R2) for 128 samples was 0.89 in the comparison of the methods, and the concordance ratio was 89.4%. The point-of-care test was shown to be a reliable alternative to the ELISAs.
Recently, a new quantitative enzyme immunoassay, the EliA™ calprotectin assay, was developed, and Oyaert et al.22 compared this assay with the point-of-care test among 183 patients with suspected IBD. Test performance of the EliA™ calprotectin assay was statistically equivalent to that of the point-of-care test.
Another quantitative enzyme immunoassay, the RIDASCREEN® calprotectin assay, has not been validated in patients with suspected IBD. The present study compared these three fecal calprotectin kits to evaluate their initial diagnostic ability in patients with IBD, IBS, or "other colitis."
All three fecal calprotectin kits were superior to CRP in distinguishing IBD from IBS or "other colitis." Overall accuracy for differentiating IBD from IBS or "other colitis" was the best for Quantum Blue® Calprotectin (97%/91%), followed by EliA™ Calprotectin (94%/89%) and RIDASCREEN® Calprotectin (84%/76%).
Some articles about a comparison of calprotectin test kits were published recently. Mirsepasi-Lauridsen et al.24 compared three ELISA calprotectin tests among IBD patients and reported that the specificity of CALPRO, EK-CAL, and HK325 is 96%, 74%, and 28%, respectively. Therefore, the three calprotectin assays tested in the present study are comparable to an ELISA for diagnostic purposes. Labaere et al.25 compared three rapid immunochromatographic assays (including Quantum Blue®), two ELISAs, and one automated immunoassay (EliA™) in terms of diagnosis and follow-up of IBD. Sensitivity and specificity for the diagnosis of IBD were 83% and 68% for Quantum Blue® and 75% and 95% for EliA™, respectively. The present study revealed similar clinical performance.
On the other hand, efficacy of fecal-calprotectin kits does not seem to be satisfactory in discriminating IBD from the "other colitis" group, considering the low specificity observed (all 40%). This is probably because the present study has a small sample size, and the study population is heterogeneous in terms of diagnosis and severity of the diseases. If the sample size of the "other colitis" group were larger, then the accuracy of fecal calprotectin kits might decrease as reported in another study.26 The three fecal calprotectin kits were not statistically helpful in discriminating between IBS and "other colitis" although calprotectin levels of patients with "other colitis" were found to be numerically higher than those of patients with IBS, as reported in other studies.2226
Among patients with CD, results of Quantum Blue® Calprotectin and EliA™ Calprotectin correlated significantly with CDAI. The RIDASCREEN® calprotectin data, however, did not correlate with CDAI, possibly because the upper limit of RIDASCREEN® Calprotectin is lower than that of the other two tests. Among patients with UC, only EliA™ Calprotectin correlated significantly with the Mayo score. EliA™ Calprotectin appeared to better reflect disease activity in patients with UC than in patients with CD. Costa et al.27 reported that fecal calprotectin shows a higher correlation with disease activity of UC than with that of CD.
We also evaluated the correlation between fecal calprotectin levels and disease location in patients with IBD. There was a significant difference in calprotectin levels between CD patients with ileocolonic involvement and those with ileal involvement. This result is consistent with a report by Schoepfer et al.,28 who studied the correlation of fecal calprotectin with the simple endoscopic score for CD among 140 patients with CD. Nevertheless, a comparison of colonic involvement with other affected locations must be confirmed in studies with a larger sample size because only one patient in the present study had colonic involvement.
The cost of these three calprotectin kits is $20−33 per sample tested (Quantum Blue®>EliA™>RIDASCREEN®). Nonetheless, the final cost can vary depending on the number of samples tested at a time because each kit is packaged differently (25 tests/kit for Quantum Blue®, 48 tests/kit for EliA™, and 96 tests/kit for RIDASCREEN®).
Finally, when we evaluated the correlation between different calprotectin kits, the correlation coefficients were high, but when we performed regression analysis, significant differences were observed. This finding is in line with the data reported in other studies.2529 Therefore, as authors of those studies pointed out, direct comparison of absolute calprotectin levels between different kits is inappropriate, and the use of the same kit is desirable during the follow-up of patients with IBD.
This study has several limitations. First, this study has a relatively small sample size. Therefore, larger studies will be necessary to confirm our results. Second, colonic involvement was present in only one patient with CD, and this situation limited the comparison between colonic involvement and other affected locations. Nevertheless, the trends and results of analysis regarding correlation of fecal calprotectin with the disease location are consistent with data from other studies.21
In conclusion, fecal calprotectin tests are useful for distinguishing IBD from IBS or "other colitis" (in the descending order of performance): Quantum Blue® Calprotectin, EliA™ Calprotectin, and RIDASCREEN® Calprotectin.
Notes
Financial support: This study was supported in part by the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) and funded by the Ministry of Health and Welfare, Republic of Korea (grant numbers: A111428, HI13C1345, and HI12C0130). It was also supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A1008096).
Conflict of interest: None.