Clinical outcome of endoscopic management in delayed postpolypectomy bleeding
Article information
Abstract
Background/Aims
The clinical course after endoscopic management of delayed postpolypectomy bleeding (DPPB) has not been clearly determined. This study aimed to assess clinical outcomes after endoscopic hemostasis of DPPB and evaluate risk factors for rebleeding after initial hemostasis.
Methods
We reviewed medical records of 198 patients who developed DPPB and underwent endoscopic hemostasis between January 2010 and February 2015. The performance of endoscopic hemostasis was assessed. Rebleeding negative and positive patients were compared.
Results
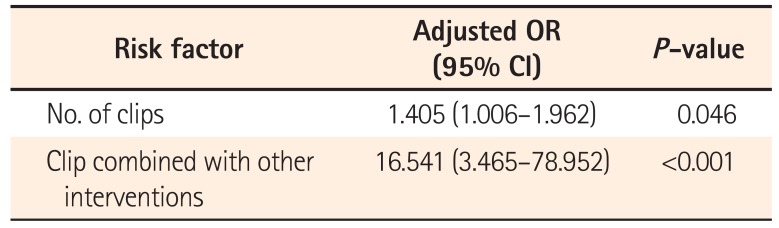
DPPB developed 1.4±1.6 days after colonoscopic polypectomy. All patients achieved initial hemostasis. Clipping was the most commonly used technique. Of 198 DPPB patients, 15 (7.6%) had rebleeding 3.3±2.5 days after initial hemostasis. The number of clips required for hemostasis was higher in the rebleeding positive group (3.2±1.6 vs. 4.2±1.9, P=0.047). Combinations of clipping with other modalities such as injection methods were more common in the rebleeding positive group (67/291, 23.0% vs. 12/17, 70.6%; P<0.001). Multivariate analysis showed a large number of clips and combination therapy were independent risk factors for rebleeding. All the rebleeding cases were successfully managed by repeat endoscopic hemostasis.
Conclusions
Endoscopic hemostasis is effective for the management of DPPB because of its high initial hemostasis rate and low rebleeding rate. Endoscopists should carefully observe patients in whom a large number of clips and/or combination therapy have been used to manage DPPB because these may be related to the severity of DPPB and a higher risk of rebleeding.
INTRODUCTION
Most colorectal cancers develop from adenomatous polyps. Colonoscopic polypectomy can remove most colorectal polyps effectively, and reduce the risk of colorectal cancer.123 Despite its effectiveness in the prevention of colorectal cancer, polypectomy is not completely safe because it is associated with complications such as bleeding and perforation.4
Postpolypectomy bleeding occurs in 0.3% to 6.1% of colonoscopic polypectomy cases.5678910 Postpolypectomy bleeding is generally classified as either immediate/early postpolypectomy bleeding (IPPB) or delayed postpolypectomy bleeding (DPPB). IPPB is usually defined as bleeding that develops immediately after resection of polyps during the colonoscopy procedure. Because endoscopists can directly detect IPPB, most cases can be managed endoscopically during the colonoscopic procedure.6 DPPB is defined as bleeding that develops after the end of colonoscopic polypectomy. Most DPPB is detected when patients complain of hematochezia several hours to several days after colonoscopic polypectomy. The incidence of DPPB is reported to be 0.2% to 2.2%.6781112 Although most endoscopists apply endoscopic hemostasis to manage DPPB, clinical outcomes of endoscopic management have not been thoroughly investigated. Thus, the aim of our study was to assess the clinical outcomes after endoscopic hemostasis for DPPB. We also evaluated the frequency of DPPB and risk factors for recurrence of bleeding after initial endoscopic hemostasis.
METHODS
1. Patients
All patients who underwent colonoscopic and/or sigmoidoscopic bleeding control for DPPB at Asan Medical Center between January 2010 and February 2015 were included in this study. DPPB was defined as hematochezia and/or melena occurring within 14 days of colonoscopic polypectomy. Techniques included cold snare polypectomy, injection assisted polypectomy (endoscopic mucosal resection, EMR), endoscopic piecemeal mucosal resection (EPMR), endoscopic submucosal resection (ESD), and ESD with snaring (hybrid ESD). Patients were categorized into two groups based on the occurrence of rebleeding. The rebleeding positive group was defined as those who presented with hematochezia and/or melena after the initial successful endoscopic hemostasis and required repeat hemostatic interventions. The rebleeding negative group was defined as those who did not show further hematochezia and/or melena after the initial successful endoscopic hemostasis.
2. Review of Clinical Data
We retrospectively reviewed medical records and endoscopy reports with pictures. Demographic data such as age, gender, laboratory findings, comorbidities, and use of medications including antiplatelet agents (aspirin, clopidogrel) and anticoagulants (warfarin, heparin) were investigated. Colonoscopic features of each resected polyp such as the size, location, endoscopic morphology, histological diagnosis, and colonoscopic polypectomy methods were analyzed. Information on endoscopic hemostasis such as the endoscopist's experience (staff vs. fellow) and the endoscopic hemostasis methods were also reviewed. Clinical outcomes after endoscopic hemostasis, including success or failure of endoscopic hemostasis, complications, recurrent bleeding, and performance of repeat interventions, were further investigated. Success of endoscopic hemostasis was defined as the cessation of bleeding after endoscopic interventions such as clipping. The Institutional Review Board of our center approved the protocol of this study.
3. Statistical Analysis
Statistical analyses were performed by using SPSS version 21.0 (IBM Corp., Armonk, NY, USA). Continuous variables are reported as means with SDs and compared using Student t-test. Categorical data were analyzed using Fisher exact test. Multivariate logistic regression analyses were performed to investigate the risk factors for recurrent bleeding after initial endoscopic hemostasis. A P-value <0.05 was considered statistically significant.
RESULTS
1. Baseline Characteristics and Clinical Course
A total of 21,780 patients underwent colonoscopic polypectomy at our institution between January 2010 and February 2015. Of these, 198 patients (0.9%) developed DPPB, which was associated with 308 polyps (Fig. 1). The mean age of the 198 patients was 60.1±11.2 years and 150 were male. EMR was the most common polypectomy method (245/308, 79.5%). DPPB developed 1.4±1.6 days (median, 0.8 days; range, 0.2–11.0 days) after colonoscopic polypectomy. Endoscopic hemostasis was attempted on the 308 polyps. A clip with or without other intervention was the most commonly used hemostatic method (263/308, 85.4%). Endoscopic hemostasis was successful for all 308 polyps in 198 patients (100%). There were no adverse events and no patient required a blood transfusion. Of the 198 patients, 17 polyps (5.5%) in 15 cases (7.6%) presented with rebleeding 3.3±2.5 days (median, 2.1 days; range, 0.8–10.0 days) after initial endoscopic hemostasis (Fig. 2). Only one additional repeat endoscopic hemostasis session was necessary to control rebleeding in 11 of the 15 patients (73.3%). Two additional sessions were required in one patient because of repeated bleeding. Three repeat endoscopic interventions were necessary in one patient. Four repeat endoscopic hemostatic procedures were performed in the remaining two patients. All 15 patients with recurrent bleeding were managed successfully without adverse events by repeat endoscopic intervention and none of the patients with rebleeding required further angiography or surgery to control recurrent bleeding. No blood transfusions were necessary in any of the rebleeding patients.

Flow diagram of patients with delayed postpolypectomy bleeding (DPPB). All DPPB patients were eventually managed by endoscopic hemostasis.

Rebleeding after initial endoscopic hemostasis in delayed postpolypectomy bleeding (DPPB). (A) Active blood oozing is noted at a large postpolypectomy ulcer where five clips were applied during a previous endoscopy to control DPPB. All five clips were attached at the periphery of the ulcer. (B) Hemostasis was achieved by the application of additional argon plasma A B coagulation.
2. Comparison between Rebleeding Positive and Negative Groups
Demographic characteristics were compared in the rebleeding positive and negative groups, and showed no significant differences regarding age, gender, laboratory findings, medications, and comorbidities (Table 1). Endoscopic features at the time of colonoscopic polypectomy were compared in the two groups (Table 2). The mean size of polyps tended to be larger in the rebleeding positive group, but this was not statistically significant (12.2±13.3 mm vs. 18.0±18.5 mm, P=0.217). There was no significant difference between the groups with respect to polyp distribution or morphology. EPMR was more frequently used in the rebleeding positive group than the rebleeding negative group (17/291, 5.8% vs. 4/17, 23.5%). Adenocarcinoma was more common in the rebleeding positive group than the rebleeding negative group (Table 2). The performance of endoscopic hemostasis was compared in the rebleeding positive and negative groups (Table 3). Clipping with or without other intervention was the most commonly used hemostatic method in both groups. The number of clips required for successful hemostasis was higher in the rebleeding positive group (3.2±1.6 vs. 4.2±1.9, P=0.047). Clipping alone was more common (183/291, 62.9% vs. 2/17, 11.8%; P<0.001) in the rebleeding negative group, whereas a combination of a clip and other modalities such as injection methods were more commonly used in the rebleeding positive group (67/291, 23.0% vs. 12/17, 70.6%; P<0.001). The experience of the endoscopists (staff vs. fellow) did not differ between the two groups.

Per-Patient Comparisons of Demographic Characteristics in the Rebleeding Positive and Negative Groups
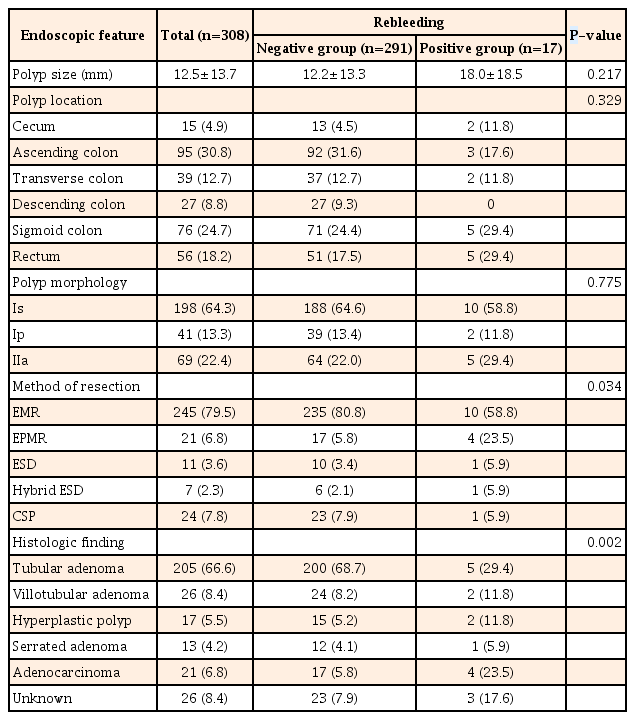
Per-Polyp Comparisons of Endoscopic and Histological Features in the Rebleeding Positive and Negative Groups
3. Risk Factors for Rebleeding after Endoscopic Hemostasis of DPPB
Multivariate analysis was performed to investigate independent risk factors for recurrent bleeding after successful endoscopic hemostasis of DPPB. A large number of clips and a combination of a clip with other hemostatic methods increased the risk of rebleeding after endoscopic hemostasis of DPPB (Table 4).
DISCUSSION
Our study showed that endoscopic interventions achieved 100% hemostasis for DPPB without any adverse events. Only 7.6% of patients who initially achieved endoscopic hemostasis developed rebleeding, and all of these patients were managed successfully by repeat endoscopic hemostasis without the additional need for other interventions such as surgery or angiographic embolization. Therefore, endoscopic hemostasis is effective and safe for the management of DPPB.
In our study, clinically significant DPPB requiring endoscopic interventions developed in 0.9% of patients who underwent colonoscopic polypectomy. This frequency corresponds to the DPPB rate of up to 2.2% reported in previous studies.69 Initial endoscopic hemostasis was achieved in all of these DPPB patients by using several hemostatic methods, with the clip method most commonly used. A previous retrospective study assessed the effectiveness of clipping in 45 cases of IPPB, 18 cases of DPPB, and nine cases of post-biopsy bleeding.13 All cases of IPPB and postbiopsy bleeding and all but one DPPB case were successfully managed by clipping. Bleeding was controlled by using a clip in combination with the placement of a detachable snare in a patient in whom clipping alone failed to achieve hemostasis. Another case series of 42 patients with postpolypectomy bleeding evaluated the usefulness of the clip method and found that initial hemostasis was successful in all patients with active bleeding.14 The average number of clips used was 2.9 in this study. Sorbi et al.9 also found that clipping was effective for the management of DPPB and that an average of 4.6 clips was required in patients with severe DPPB who required hospitalization. In our study, the average number of clips needed for initial hemostasis of DPPB was 3.3, which is in agreement with previous studies. Besides clipping, other methods were used for hemostasis in our study, including injection of epinephrine and/or fibrin glue, argon plasma coagulation, and coagulation forceps. Methods other than clipping were more frequently used in the rebleeding positive group. The choice of hemostatic method apparently depends on an individual endoscopist's preference.1215 A combination of several methods was required in some cases. Because this was not a prospective study, we could not compare the hemostatic methods. Nonetheless, we suggest that clipping may be a better hemostatic method for most DPPB because it can ensure mechanical closure of the bleeding vessels regardless of whether they are arteries or veins. In contrast, other methods such as injection and/or thermal coagulation may be effective for venous oozing but not for arterial spurting, and may cause perforation of thin walled postpolypectomy ulcers.16 This might be the reason why clipping was the most commonly used hemostatic technique in our study of DPPB cases.
Only 5.5% of polyps associated with DPPB showed rebleeding after initial endoscopic hemostasis. In per-patient analysis, only 7.6% of DPPB patients showed rebleeding. Previous studies reported a rebleeding rate of 4.2% to 9.5%,913 which is comparable to the rate reported in this study. Although this rebleeding rate is not negligible, it is not significantly high. Therefore, endoscopic hemostasis is effective for the management of DPPB because it achieves a 100% initial hemostasis rate, and also results in a low rebleeding rate.
A large number of clips and a combination of clipping with other interventions were independent risk factors for rebleeding after initial hemostasis in DPPB. We believe that large numbers of clips and combination therapy may indicate technical difficulties during the hemostatic procedure. In addition, unfamiliarity with clipping may increase the number of clips required. A previous study revealed that operator familiarity with clipping had a bearing on successful hemostasis.15 Furthermore, the number of clips used was related to the appearance and severity of DPPB. Spurting arterial bleeding required more clips to achieve hemostasis than oozing bleeding, and active bleeding required more clips than non-bleeding visible vessels.14 These considerations suggest that in severe, active DPPB cases, in which there is a high degree of difficulty in obtaining endoscopic hemostasis, more clips may be required and combination therapy may be needed more frequently, which can be associated with a high risk of rebleeding. Therefore, these patients should be more closely monitored, even when initial hemostasis for DPPB has been achieved.
Several factors reportedly associated with a higher risk of DPPB, such as anticoagulation, comorbidities, large polyp size, and inexperience of the endoscopists,9171819 were not associated with the risk of rebleeding after initial hemostasis of DPPB in our study. We suggest that the quality of initial hemostasis may be the most important factor affecting the recurrence of bleeding.
Our study has several limitations. First, it was retrospective. Therefore, we could not analyze in detail the factors involved in DPPB. For example, we could not assess the nature of DPPB in some cases, i.e., whether the bleeding was arterial or venous. Second, we did not include patients with minimal DPPB who were managed conservatively, or unstable patients who were initially managed by angiographic embolization because of severe DPPB. Therefore, we could not completely determine the usefulness of endoscopic hemostasis in all DPPB patients. Third, other than rebleeding after endoscopic hemostasis, our study did not evaluate other aspects of DPPB management. We did not evaluate bowel preparation methods for DPPB patients and timing of diet resumption after endoscopic hemostasis. In addition, we did not determine whether admission was necessary in the management of DPPB. If DPPB management is not effective, a variety of issues such as medicolegal problems and high cost may develop. Therefore, further studies addressing all these aspects are necessary to provide more useful information on the management of DPPB. Fourth, we did not analyze the initial DPPB rates according to endoscopic resection method in all 21,780 patients. Thus, we could not assess the DPPB rate after cold snare polypectomy, EMR, EPMR, ESD, and hybrid ESD in this study. Finally, this was a single, tertiary center experience, and caution should be exercised when generalizing the findings of our study to other situations.
In conclusion, endoscopic hemostasis was very effective for the management of DPPB because it achieved 100% initial hemostasis and had a low rebleeding rate. Because a large number of clips and clipping combined with other endoscopic interventions are risk factors for rebleeding after initial hemostasis in DPPB, these patients require closer monitoring. Additional trials of methods to further lower the rebleeding rate should be performed in the future.
Notes
Financial support: None.
Conflict of interest: None.