 |
 |
- Search
| Intest Res > Volume 17(2); 2019 > Article |
|
Abstract
Background/Aims
Methods
Results
NOTES
FINANCIAL SUPPORT
The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST
T.K. received lecture fees from Mitsubishi Tanabe Pharma Co., Ltd, Eisai Co., Ltd, Kyorin Pharmaceutical Co., Ltd, AbbVie Inc., Janssen Pharmaceutical K.K, JIMRO Co., Ltd, Ajinomoto Pharma Co., Ltd, EA Pharma Co., Ltd, Astellas Pharma Inc, Mochida Pharmaceutical Co., Ltd, Asahi Kasei Medical Co., Ltd, Takeda Pharmaceutical Co., Ltd, Gilead Sciences Inc., Celltrion Inc, Nippon Kayaku Co., Ltd, Alfresa Pharma Co., Ltd and advisory/consultancy fees from Janssen Pharmaceutical K.K, Pfizer Inc, Kyorin Pharmaceutical Co., Ltd, Mochida Pharmaceutical Co., Ltd, Takeda Pharmaceutical Co., Ltd, Eli Lilly and Company, Ferring Pharmaceutical Co., Ltd, Nippon Kayaku Co., Ltd, Thermo Fisher Scientific Inc, Covidien Japan Inc and research grant from EA Pharma Co., Ltd, Thermo Fisher Scientific Inc, Alfresa Pharma Co., Ltd. T.H. received lecture fees from AbbVie Inc, Kyorin Pharmaceutical Co., Ltd, Eisai Co., Ltd, Mitsubishi Tanabe Pharma Co., Ltd, EA Pharma Co., Ltd, JIMRO Co., Ltd and ZERIA Pharmaceutical Co., Ltd. M.N. received lecture fees from Takeda Pharmaceutical Co. Ltd and Mochida Pharmaceutical Co., Ltd. However, all of these are not relevant to this article.
ACKNOWLEDGEMENTS
Fig. 1.
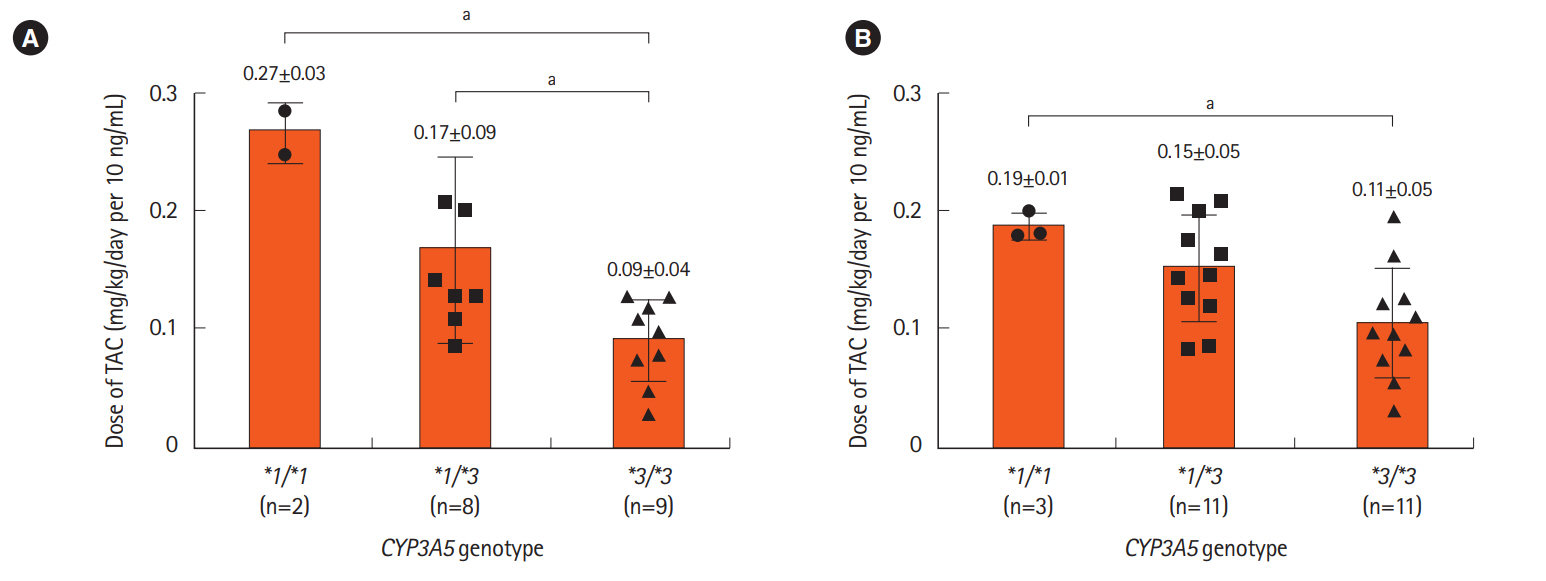
Fig. 2.
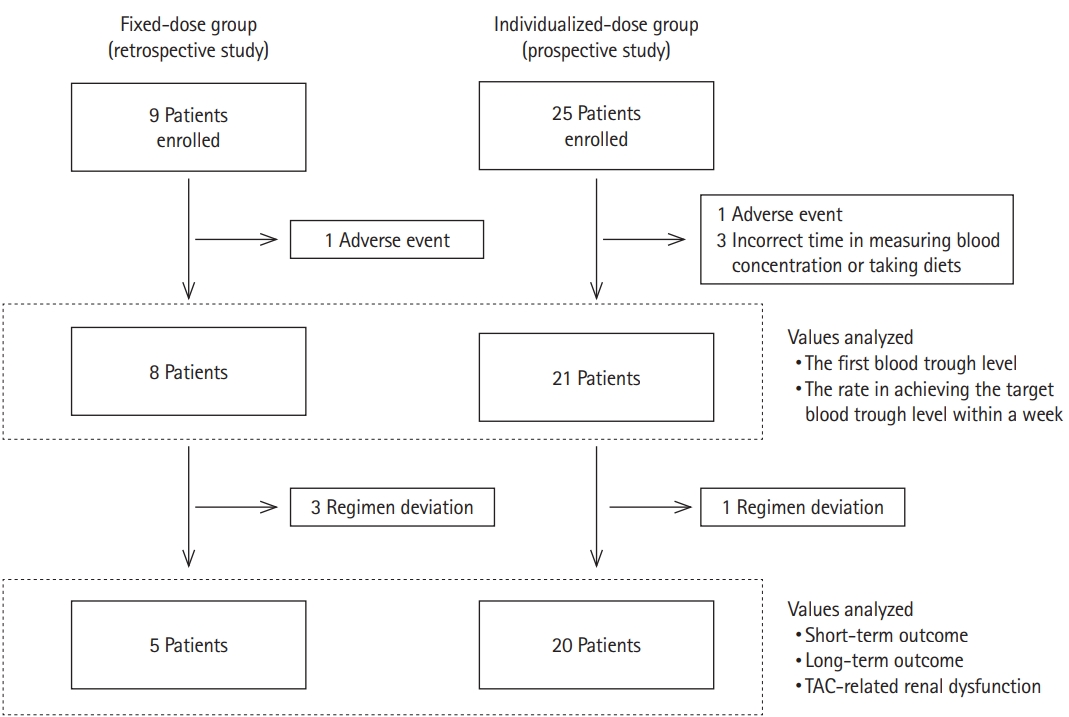
Fig. 3.
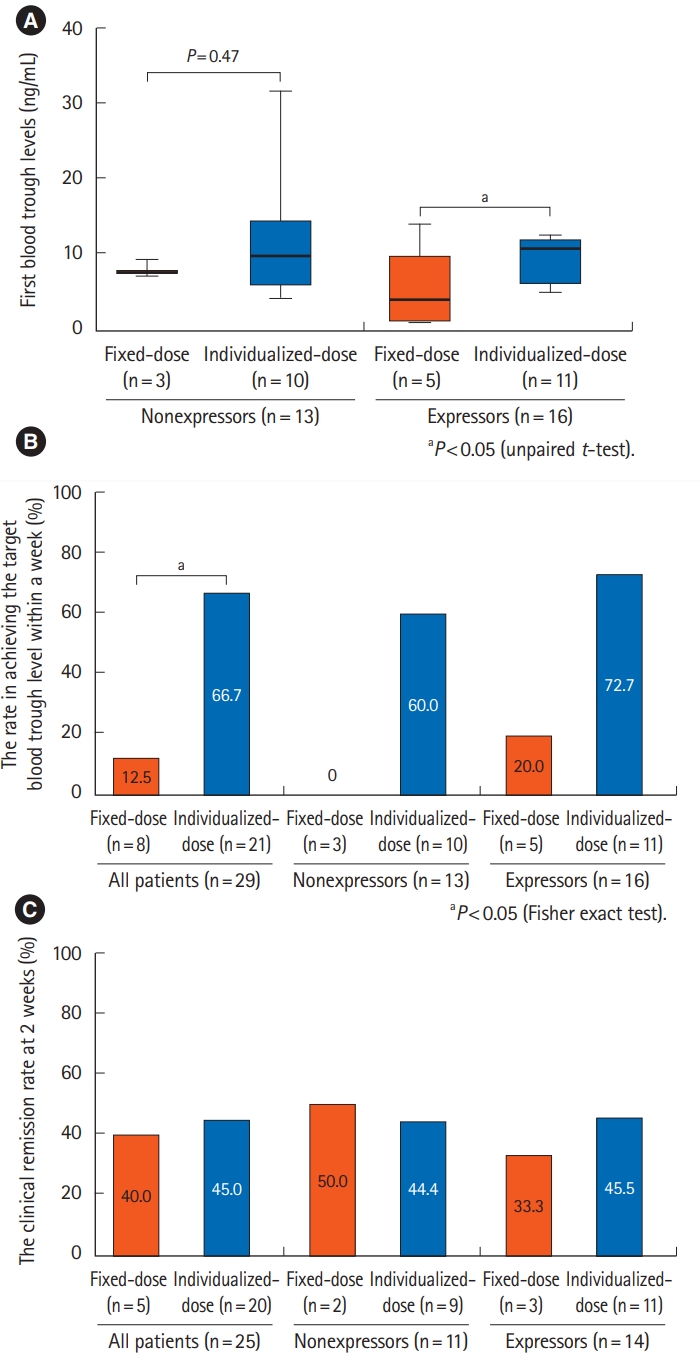
Fig. 4.
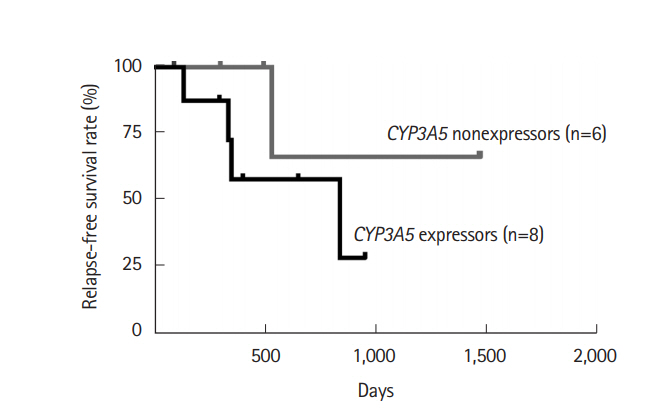
Fig. 5.
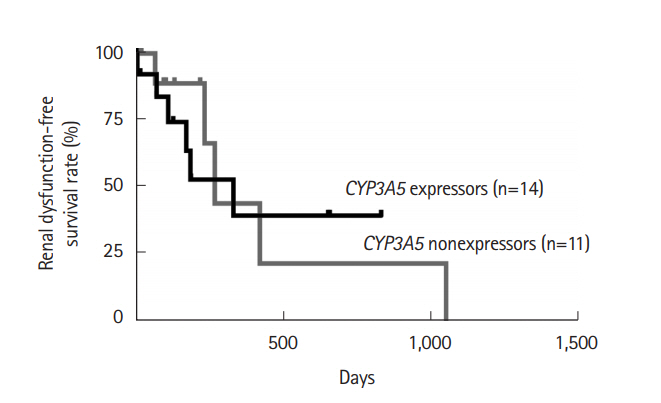
Table 1.
| Characteristic | Fixed-dose (n=8) | Individualizeddose (n=21) | P-value |
|---|---|---|---|
| Age (yr) | 35.5±3.1 | 38.6±3.5 | 0.61a |
| Male sex | 5 (62.5) | 14 (66.7) | 1.00b |
| Body weight (kg) | 57.8±4.3 | 55.0±2.0 | 0.51a |
| Disease duration (mon) | 84.7±33.0 | 85.0±18.7 | 0.99a |
| Extent of disease | |||
| Entire colitis | 6 (75.0) | 15 (71.4) | 1.00b |
| Left-sided colitis | 2 (25.0) | 6 (28.6) | |
| CYP3A5 genotype | 0.84c | ||
| Expressor | *1/*1 | 1 (12.5) | 3 (14.3) |
| *1/*3 | 4 (50.0) | 8 (38.1) | |
| Nonexpressor | *3/*3 | 3 (37.5) | 10 (47.6) |
| Initial TAC dosage (mg/day) | 6.0±1.8 | 7.5±2.0 | 0.11a |
| Time to initial blood test (day) | 3.38±0.42 | 3.00±0.13 | 0.26a |
| No. of dose adjustment | 0.02c | ||
| 0 | 1 (12.5) | 13 (61.9) | |
| 1 | 4 (50.0) | 7 (33.3) | |
| ≥2 | 3 (37.5) | 1 (4.8) | |
| Food-intake (yes/no) | 6/2 | 13/8 | 0.67b |
| Previous use of biologic agents | 3 (37.5) | 15 (71.4) | 0.20b |
| Immunosuppressive therapy | 5 (62.5) | 14 (66.7) | 1.00b |
| Lichtiger CAI | 10.6±0.9 | 11.0±0.9 | 0.83a |
| CRP (mg/dL) | 2.58±1.26 | 4.68±1.59 | 0.43a |
| Cr (mg/dL) | 0.75±0.07 | 0.74±0.03 | 0.88a |
REFERENCES
- TOOLS








