Polypharmacy is a risk factor for disease flare in adult patients with ulcerative colitis: a retrospective cohort study
Article information
Abstract
Background/Aims
Polypharmacy is a common clinical problem with chronic diseases that can be associated with adverse patient outcomes. The present study aimed to determine the prevalence and patient-specific characteristics associated with polypharmacy in an ulcerative colitis (UC) population and to assess the impact of polypharmacy on disease outcomes.
Methods
A retrospective chart review of patients with UC who visited a tertiary medical center outpatient clinic between 2006 and 2011 was performed. Polypharmacy was defined as major ( ≥ 5 non-UC medications) or minor (2–4 non-UC medications). UC medications were excluded in the polypharmacy grouping to minimize the confounding between disease severity and polypharmacy. Outcomes of interest include disease flare, therapy escalation, UC-related hospitalization, and surgery within 5 years of the initial visit.
Results
A total of 457 patients with UC were eligible for baseline analysis. Major polypharmacy was identified in 29.8% of patients, and minor polypharmacy was identified in 40.9% of the population. Polypharmacy at baseline was associated with advanced age (P< 0.001), female sex (P= 0.019), functional gastrointestinal (GI) disorders (P< 0.001), and psychiatric disease (P< 0.001). Over 5 years of follow-up, 265 patients remained eligible for analysis. After adjusting for age, sex, functional GI disorders, and psychiatric disease, major polypharmacy was found to be significantly associated with an increased risk of disease flare (odds ratio, 4.00; 95% confidence interval, 1.66–9.62). However, major polypharmacy was not associated with the risk of therapy escalation, hospitalization, or surgery.
Conclusions
Polypharmacy from non-inflammatory bowel disease medications was present in a substantial proportion of adult patients with UC and was associated with an increased risk of disease flare.
INTRODUCTION
Inflammatory bowel disease (IBD) is an immune-mediated disease characterized by chronic inflammation of the GI tract and has a course characterized by relapse and remission [1]. Over 1.5 million Americans and 2.2 million Europeans are known to have either CD or UC and the global prevalence is increasing [2]. As a life-long disease, IBD may have a negative impact on quality of life and can be associated with a variety of co-morbidities [3]. A significant portion of patients with IBD are known to have sleep disturbances, depression, anxiety, and chronic pain [4-6]. IBD is also associated with the development of other autoimmune diseases, thromboembolism, and colon cancer [7-9]. In addition, diseases common in the general population, such as coronary heart disease, diabetes, and asthma, are also prevalent in the IBD population [10], and with the expanding aging population a growing number of IBD patients also live with other chronic diseases [11]. The concurrent management of these conditions along with IBD has thus become a more complicated task facing primary care physicians and gastroenterologists.
One issue involved in the management of IBD patients is to mitigate the risk of polypharmacy. Polypharmacy can be defined as major polypharmacy with the use of 5 or more medications at the same time [12]. The prevalence of polypharmacy in IBD has been shown to be greater than 20% [13]. Compared to the general population, IBD patients have been shown to consume more analgesic medications, antidepressants, and anxiolytics, as well as high rates of supplement, probiotic, and complimentary therapies which contribute to this risk [14]. Polypharmacy has been associated with poorer clinical outcomes in several chronic diseases in part due to increased risk of drug-drug interactions, along with reduced medication adherence associated with increased medication burden [15]. It has been shown that patients with CD who consumed more medications had increased disease activity and lower quality of life [16]. However, there has been limited research on the prevalence of polypharmacy in UC and the long-term effects of polypharmacy on clinical outcomes in UC.
The objectives of the present study were to estimate the prevalence of polypharmacy in an UC population, to determine patient-specific characteristics associated with polypharmacy, and to assess the longitudinal impact of polypharmacy on clinical outcomes of UC over a 5-year interval.
METHODS
1. Study Population
In this retrospective study, adult patients with an established diagnosis of UC followed at the University of Virginia Digestive Health clinic from January 1, 2006 to December 31, 2011 were identified using the electronic medical record. Patients were excluded if they had a diagnosis of CD or indeterminate colitis, were less than 18 years of age, or had incomplete medical records including missing medication lists or missing medical/surgical history. Patients were excluded from the 5-year follow-up data if they had incomplete medical data or were lost to follow-up during this time. The study was approved by Institutional Review Board at the University of Virginia.
2. Data Collection
Baseline demographic information including sex, age, smoking status and alcohol history were collected. Disease-specific information including the extent of disease based on the Montreal classification, disease duration, past and current UC treatment regimens, and medical history were recorded. Functional GI disorders, including IBS, dyspepsia, gastroesophageal reflux disease, constipation etc., and psychiatric disorders, including generalized anxiety disorder and major depressive disorder, were identified by diagnostic code in the electronic medical record. Data on medication use, including over-the-counter (OTC), supplements such as vitamins and minerals, and prescription medications that patients reported taking on a regular basis were collected from the initial clinic visit. The total number of non-UC medications, including OTC, vitamin and mineral supplements, reported by the patients was classified according to degree of polypharmacy: none (0–1 medication), minor polypharmacy (2–4 medications) and major polypharmacy (>5 medications) as previously defined [17]. Of note, UC medications were excluded in the polypharmacy grouping to minimize the confounding between disease severity and polypharmacy classes, in which patients with more severe diseases may require more than one UC medications.
3. Outcome Measurement
Clinical outcomes of interest included UC flare, UC-related hospitalization, UC-related surgery (colectomy) and UC therapy escalation within 5 years of initial clinic visit. A disease flare was identified when documented in the medical record by attending gastroenterologist and changes were made to the patient’s treatment. Therapy escalation was defined as escalation from 5-aminosalicylate (5-ASA) therapy to immunomodulator or biologic therapy, or from immunomodulator to biologic therapy. In a subgroup analysis, we analyzed the relationship between the clinical outcome mentioned above and common medication categories of interest, including opioids and NSAIDs. The online clinical database Lexicomp® (Wolters Kluwer Health, Inc. Hudson, OH, USA). Online was used to identify potential drug-drug interactions that could lead to adverse effects or negative clinical outcomes.
4. Statistical Analysis
Statistical analyses were performed using the Statistical Analysis Systems (SAS) Software program version 9.4 (SAS Institute Inc., Cary, NC, USA). The α-error was set at 0.05 and reported P-values are 2-sided. Descriptive statistics were reported as percentages, mean values, and standard errors of the mean. Univariate analysis was performed to determine independent predictors of polypharmacy by using chi-square tests for categorical variables and ANOVA for continuous variables. As for 5-year follow-up, outcome of interests include UC flare, hospitalization, surgery, and therapy escalation. A multivariate logistic regression analysis was used to assess the relationship between polypharmacy class and these clinical outcomes by adjusting for potential confounders, which were variables that were significantly associated with polypharmacy at baseline.
RESULTS
1. Baseline Characteristics by Polypharmacy Class
A total of 457 out of 498 patients with UC were eligible for baseline analysis. Major polypharmacy was identified in 136 patients (29.8%) and minor polypharmacy was seen in 187 of the population (40.9%). Polypharmacy at baseline was associated with increasing age (P<0.001) and female sex (P=0.019). Polypharmacy was associated with functional GI disorders, with 18% of patients with major polypharmacy having concomitant functional GI disorders (P<0.001). Polypharmacy was also associated with psychiatric disease, with 32% of patients with major polypharmacy having concomitant psychiatric disease (P<0.001). There was a trend between disease duration and polypharmacy, although this did not achieve statistical significance (P=0.051). Neither tobacco nor alcohol usage was associated with polypharmacy. There was also no association between polypharmacy and disease extent based on the Montreal classification (Table 1).
2. Clinical Outcomes by Polypharmacy Class in 5-Year Follow-up
Over 5 years of follow-up, 265 out of 457 patients remained eligible for data analysis. After adjusting for age, sex, functional GI disorders, and psychiatric disease, major polypharmacy was significantly associated with an increased risk of UC flare (OR, 4.00; 95% CI, 1.66–9.62). Major polypharmacy was not associated with an increased risk of therapy escalation, UC-related hospitalization or surgery (Fig. 1).
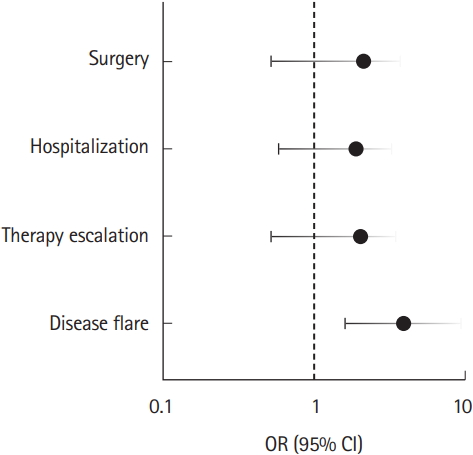
Differences in OR with 95% CI between patients in major polypharmacy class (n =75) and those without polypharmacy (n=89) were assessed using multiple logistic regression model after adjusting for age, sex, functional GI disorders and psychiatric disease. Major polypharmacy: >5 non-UC medications. Y-axis is logtransformed (P<0.05).
3. Clinical Outcomes by Medication Categories
After adjusting for age, sex, functional GI disorders, psychiatric disease and polypharmacy class, further analysis on specific medication categories showed that baseline usage of opioid pain medication was associated with increased risk of hospitalization (OR, 3.84; 95% CI, 1.09–13.57) and usage of prebiotics was associated with higher chance of therapy escalation (OR, 8.40; 95% CI, 1.44–49.03). Antidepressants, benzodiazepines, NSAIDs, and probiotic usage at baseline were not associated with any of the disease outcome measurements (Table 2).
4. Drug-Drug Interaction
A total of 407 clinically relevant drug-drug interactions as defined by Lexicomp® Class D or X were noted in the present study, with 115 of them being unique. The most common drug-drug interaction was between mesalamine and proton pump inhibitors in 51 patients, followed by mesalamine and calcium supplements in 40 patients. The most common clinically significant drug-drug interactions are listed in Table 3.
DISCUSSION
Approximately one-third of UC patients in our cohort were found to have major polypharmacy, defined as 5 or more non-UC medications. This proportion is similar to those previously published in the literature. One study evaluating geriatric patients with IBD reported major polypharmacy in 22% of the patient population [13], whereas approximately half of patients were taking 5 or more medications in a study in CD [13,16]. An association between polypharmacy and patients who were older, female, had functional GI disorders or concomitant psychiatric disease has also been found in previous studies involving IBD and other chronic diseases [18,19]. Our study is different than previous research in that only non-IBD medications were included in the polypharmacy grouping [16]. This approach is suitable for our study design, because it can help avoid some confounding from disease severity, in which patients with more severe UC may be taking more IBD drugs and the current study is lacking data on clinical disease activity or endoscopic scores. Including the non-IBD medications here may also be more clinically relevant, as the IBD medications may be less likely to be weaned off, compared to some other non-IBD medications.
The high prevalence of polypharmacy in UC is not a trivial issue, as polypharmacy has been associated with a variety of adverse clinical outcomes in chronic disease [20]. However, research investigating the long-term impact of polypharmacy on clinical outcomes in UC has been limited. The current study demonstrated that polypharmacy at baseline was associated with an increased risk of UC disease flare. Potential reasons for this association include decreased medication adherence, increased adverse effects from drug-drug interactions and possible negative impact on gut microbiome. As for decreased adherence, research has suggested that polypharmacy could lower treatment compliance by increasing drug burdens in patients with chronic diseases, such as those with chronic obstructive pulmonary diseases and systemic lupus erythematosus [21-23]. Given the lack of adherence data in our cohort, it is difficult to assess whether lower adherence is a primary driver behind the relationship between polypharmacy and disease flare. However, other factors, in addition to low medication adherence, might also play important roles. Studies have shown that polypharmacy might increase the risk of adverse events from drug-drug interactions [24-26]. For example, our study identified 51 cases of potential interactions between mesalamine and proton pump inhibitors; given the pH-dependent release of several mesalamine formulations, this could result in the premature release of the active drug in the small bowel and potentially diminish mesalamine’s therapeutic effect [27]. In addition to medical adherence and drug interactions, polypharmacy also has the potential to alter the composition of the gut microbiome. Recent data suggests that gut bacteria are sensitive to a variety of nonantibiotic medications, including antipsychotics and anti-diabetic medications [28]. This in theory could contribute to intestinal dysbiosis and potentially increase the risk of disease flare. This hypothesis will need to be investigated in future studies.
The current study did not identify a statistically significant association between polypharmacy and therapy escalation, hospitalization or surgery. The lack of significance may be limited by the relatively small sample size. It is also possible that some of the disease flares were controlled with adjustments in 5-ASA therapy, negating the need for therapy escalation, hospitalization or surgery. Future studies with a larger cohort of patients may further clarify such relationship.
In our UC cohort, opioid use was associated with a higher risk of UC-related hospitalization. Along with the opioid epidemic nationally, the issue of opioid usage in IBD has received increased attention in recent years. Opioid use in the IBD population is unfortunately common, and is associated with higher rates of Emergency Department visits and hospital utilization [29,30]. Despite significant advancements in UC therapy with the introduction of biologic therapy, data suggest that the prevalence of opioid use in patients with IBD has not changed significantly during this time [31]. There are clear negative effects of opioid use in the IBD population, and given that the discontinuation of opioids has the potential to improve patient outcomes including medication adherence and reduce disease activity [32], it is important to address opioid usage during medication reviews at patient clinic visits.
The current study did not identify an association between the use of psychiatric medications with the 5-year clinical outcomes of interest. IBD patients with psychiatric illness have been shown to have higher rates of GI symptoms and worse clinical outcomes in other studies [33,34]. A lack of a relationship between psychiatric medication and negative clinical outcomes may suggest that psychiatric illness was adequately managed in this patient cohort, so that the negative impact of psychiatric illness was offset by the potential benefits of effective medical therapy. Previous research has shown that antidepressants, when prescribed to treat concomitant mood disorders in IBD, may be associated with lower rates of clinical relapse and steroid use [35]. In addition, non-pharmacologic therapies including psychological counseling have been shown to improve the clinical course of IBD [36], and are available to patients in our clinic population, which could contribute to better clinical outcomes. The current study also did not identify an association between the use of NSAID at baseline and clinical outcomes of UC in 5 years. The connection between NSAID and IBD remains inconclusive to date based on published literature. Some studies have suggested that NSAIDs are associated with clinical relapse of quiescent IBD [37-39] and may increase the risk of hospitalization [40]. However, other studies have shown no association between IBD flare and the use of NSAIDs [41,42]. It also remains unclear whether COX2 selective inhibitors are safer than conventional NSAIDs in IBD. One randomized controlled trial has suggested that short-term usage of celecoxib was safe in treating arthritis in UC patients and did not result in higher relapse rate than placebo [43]. On the other hand, an open-label trial showed the opposite, in which all patients experienced a flare of IBD within 6 weeks of initiating COX2 therapy and 38% of them had resolution of their symptoms upon discontinuation of the treatment [44]. A recent meta-analysis has concluded that there is insufficient data to determine the impact of COX2 inhibitors on IBD exacerbations [45]. Therefore, the connection between NSAID and IBD remains inconclusive to date and further studies are needed to clarify this association.
The present study has several limitations. As a retrospective cohort study, the findings support an association between polypharmacy and risk of UC flare, but not causation, and may not be generalizable to the UC population as a whole. Another limitation is that clinical disease activity and endoscopy scores were not consistently recorded in the medical record and thus not included in the study. However, in order to reduce the confounding of disease severity on polypharmacy, patients were categorized the polypharmacy class based on non-UC medications, so that patients taking more UC medications would not automatically fall into a major polypharmacy group. Considering patients with more active disease may consume more GI symptomatic drugs, we also performed a subgroup analysis by excluding GI symptomatic drugs in the polypharmacy group. This analysis demonstrated that major polypharmacy was still associated with higher risk of disease flare (OR, 5.92; 95% CI, 2.10–16.71). In addition, the study adjusted for other common covariates related to symptom severity, including functional GI disorders and psychiatric disease. While this study did not incorporate disease duration into the multivariate analysis given its lack of statistical significance at baseline (P=0.051), including disease duration in the multivariate model increased the OR between polypharmacy and disease flare from 4.0 to 5.3 (data not shown), which would be unlikely if polypharmacy and disease flare were confounded by disease severity. Furthermore, the study had a relatively high proportion of patients that were lost to follow-up at 5 years. While this could lead to recruitment bias from differential loss to follow-up, the proportion of each polypharmacy class were largely unchanged at 5 years (major 21.5%, minor 42.6%, no polypharmacy 35.8%), suggesting loss to follow up was similar across groups and did not favor one group over another. In addition, the current study is limited by the lack of data on medication adherence, which may represent an important contributing factor for the association between polypharmacy and disease flare. Finally, certain therapy classes including supplements and complimentary therapies may have been inconsistently documented in the electronic medical record. While in this cohort there was a positive association between prebiotic usage and therapy escalation, interpretation of this finding is limited by what was likely suboptimal reporting of prebiotics in the electronic medical record and the small number of patients reporting prebiotic use.
As the first study to evaluate the longitudinal clinical impact of polypharmacy in UC, our cohort demonstrated that polypharmacy from non-IBD medications was present in a substantial proportion of adult patients and was independently associated with an increased risk of UC flare. The findings suggest that clinicians should be aware of polypharmacy in UC management and play a proactive role in minimizing unnecessary medications in this patient population.
Notes
FINANCIAL SUPPORT
The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION
Conceptualization: Behm BW. Data curation: Wang J, Nakamura TI. Formal analysis: Wang J. Methodology: Behm BW, Wang J. Project administration: Behm BW, Tuskey AG. Visualization: all authors. Writing - original draft: Wang J. Writing - review & editing: Wang J, Nakamura TI, Tuskey AG, Behm BW. Approval of final manuscript: all authors.



