|
 |
- Search
| Intest Res > Volume 18(3); 2020 > Article |
|
Abstract
Background/Aims
Inflammatory bowel disease (IBD) involves chronic inflammation of the colon with ulcerative colitis (UC), and the colon and/or small intestine with Crohnās disease (CD). Pneumatosis intestinalis (PI), characterized by compromise of the intestinal wall with gas-filled cysts, has rarely been reported with IBD. The presentation, best management and outcomes of PI with IBD are poorly defined.
Methods
We conducted a search for PI in all abdominal computed tomography (CT) reports at 2 large tertiary care hospitals from January 1, 2010 to December 31, 2017, cross referenced to ICD codes for IBD. CT and chart review was performed to confirm PI and IBD respectively. A systematic review excluding case reports was performed for PI with IBD for comparison.
Results
Of 5,990 patients with a CT abdomen report mentioning PI, we identified 11 cases of PI with IBD, 4 UC, 6 CD, and 1 indeterminate colitis. PI was limited to the small bowel in 5 patients, the right colon in 5, and small bowel and colonic in 1. All 3 mortalities had CD, small intestinal PI and portal/mesenteric venous gas. The systematic literature search identified 9 articles describing 58 patients with IBD and PI. These cases were mostly included in larger cohorts of PI patients without extractable data on presentation or outcomes in the IBD subpopulation.
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder mainly including CD and UC. Overall, IBD affects about 1.4 million Americans with prevalence rates ranging from 25 to 300 per 100,000 persons for CD and 35 to 250 per 100,000 persons for UC [1,2]. CD can affect any portion of the GI tract, but most commonly affects the terminal ileum and/or proximal colon. CD is characterized by discontinuous, often transmural inflammation that typically spares the rectum. Conversely, UC classically involves the rectum in a pattern of continuous inflammation which tracks proximally toward the cecum, affecting mainly the mucosa [3]. The pathogenesis of IBD has yet to be fully elucidated. Nonetheless, evidence suggests that an inappropriate inflammatory response in genetically predisposed individuals is caused by dysregulation between the commensal microbiota, intestinal epithelial cells, and immune cells within these tissues [4]. The inflammatory response results in a compromised mucosal barrier, which may allow luminal, immunogenic bacteria into the lamina propria, potentially perpetuating inflammation [5].
Pneumatosis intestinalis (PI), a relatively rare disorder, is defined by multiple gas-filled cysts within the submucosa and/or subserosa of the intestine. Though palpation of an abdominal mass on physical exam may be appreciated [6], it is most commonly identified by CT imaging. There are 2 main theories as to how PI develops: mechanical and bacterial [6]. The mechanical theory postulates that intraluminal gas is forced into a defect or potential defect of the intestinal mucosa (i.e., from direct trauma or colitis) when the bowel wall is placed under pressure [7]. Gas may also be introduced to the bowel wall from the lungs, with the air first tracking through the mediastinum [8]. The bacterial theory states that cystic gas collections result from hydrogen producing bacteria within the bowel wall, and is supported by the presence of hydrogen in some cysts without an obvious connection between the mucosa [6]. Laboratory evidence both supporting and refuting this theory exists with the ultimate cause of PI still a matter of debate [9].
Some non-GI conditions that have been correlated with the appearance of PI include emphysema, AIDS (acquired immune deficiency syndrome), organ transplantation, glucocorticoid use, chemotherapy use, and collagen vascular diseases. In about one-fifth of patients, PI is considered primary, in that there is no other associated illness. Not surprisingly, PI has also been observed in patients with IBD. Most descriptions of PI occurring in the setting of IBD are to be found in case reports, which are inadequate to provide data on the prevalence and significance of PI in the setting of IBD. Given the limited available information, we sought to comprehensively review for all cases or PI and IBD encountered in our health system, examine clinical presentations, treatment and outcomes. Additionally, we conducted a systematic review of the literature of PI in the setting of IBD to better understand whether our experience was typical of this patient population.
Following IRB approval (IRB No. 18-0019), a retrospective case series was conducted of all patients over 18 years of age who underwent an emergency room or inpatient CT of the abdomen for any indication at 2 of Northwell Healthās Tertiary Care Hospitals, North Shore University Hospital and Long Island Jewish Medical Center from January 1, 2010 to December 31, 2017. Informed consent was waived for the study. All result documents/reports underwent a natural language search with mPower (Nuance Communications, Burlington, MA, USA) for the terms āpneumatosisā or āpneumatosis intestinalisā or āpneumatosis coli.ā Only those with ICD-9 or ICD-10 diagnosis of IBD were included for further review to confirm that PI was present. That is, patients whose CT report excluded PI (e.g., āno evidence of pneumatosisā) were excluded from analysis. Images were reviewed by North Shore University Hospitalās chief of abdominal imaging (G.G.). Individual charts were then reviewed for demographics, IBD type/history, treatment both prior to and following PI diagnosis, and outcomes data including length of stay and mortality.
A systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis checklist (PRISMA) guidelines. Institutional review board approval was not required.
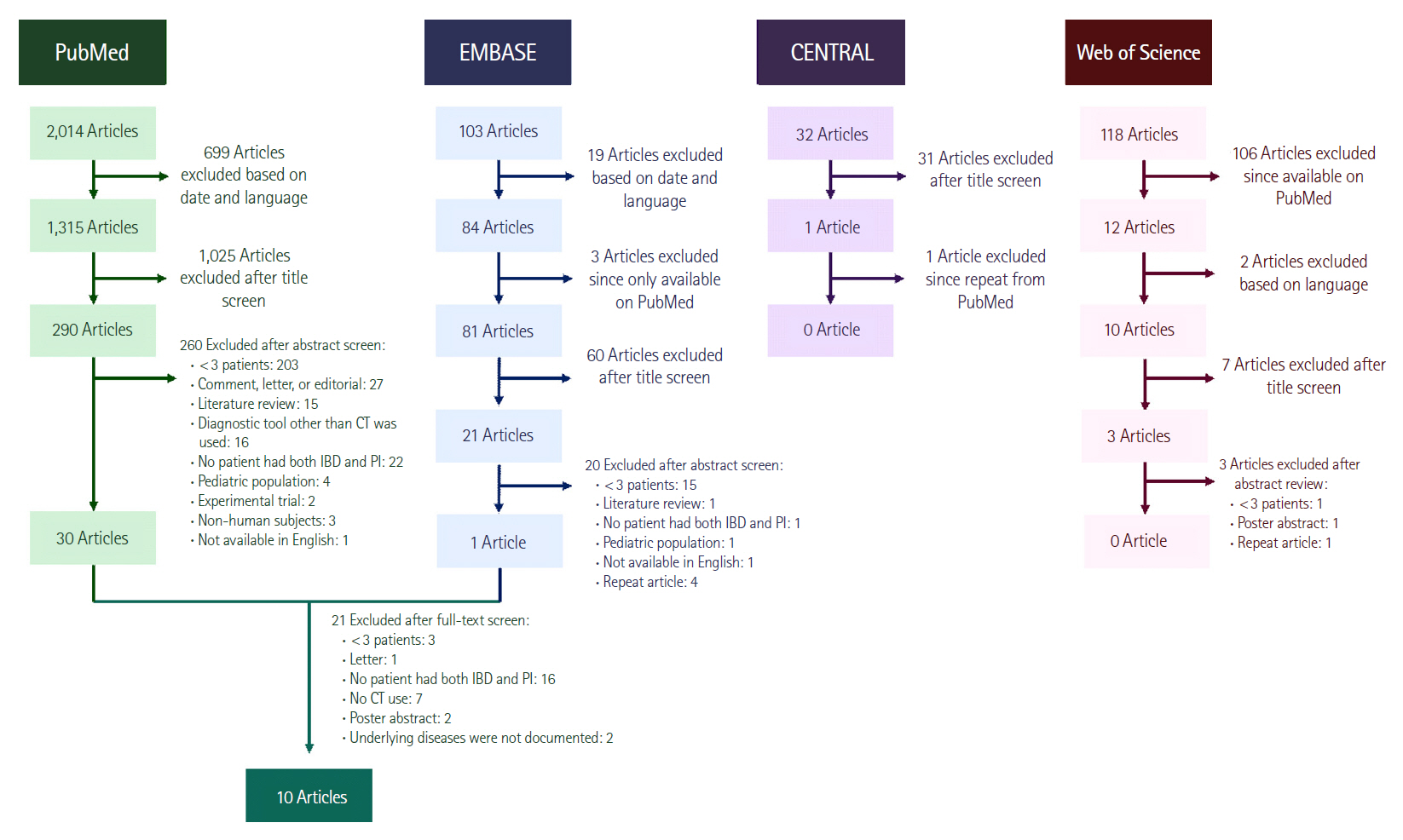
An online search for relevant publications was performed using PubMed/Medline, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science databases. For PubMed, the search conducted was: ((((((((((ulcerative colitis) OR Crohnās disease) OR inflammatory bowel disease) OR enteritis) OR āEnteritisā[Mesh]) AND āintestinal emphysemaā) OR pseudolipomatosis) OR (pneumatosis[All Fields] AND intestinalis[All Fields])) OR pneumatosis intestinalis) OR āPneumatosis Cystoides Intestinalisā[Mesh]) OR lymphopneumatosis. For the other databases, key search terms were used. For EMBASE, the terms used were āāpneumatosis intestinalisā/expā and āāinflammatory bowel diseaseā/exp.ā For CENTRAL and Web of Science, the key words were āpneumatosisā and āinflammatory bowel disease.ā Only papers published in English and between the dates January 1, 1972 to August 20, 2018 were included. The year 1972 was chosen as the cutoff date as this was the year CT scanners started being manufactured [10]. All titles were initially screened, and appropriate abstracts were reviewed. Articles identified on PubMed with appropriate titles also had their MeSH terms reviewed.
To be included in this review, studies had to meet the following publication criteria: they reported on more than 3 patients with PI and the diagnostic tool used was CT.
Studies were excluded from analysis if (1) the article was a letter, editorial, or comment; (2) they reported on pediatric patients; and (3) no IBD patients were included.
Two reviewers (K.S. and M.U.) independently reviewed the literature according to the above, predefined guidelines. Each reviewer read the identified papers to ensure that all the predefined qualities were met. The title and study details (first author, journal, year) were recorded. Within each article, the number of patients with IBD and PI were identified. Of these patients, presence of portal venous gas, type of IBD (CD, UC, IBD undefined), method of PI diagnosis, treatment (medical and/or surgical) and outcomes (mortality) were recorded. Where information was unavailable, unknown was recorded. All data were independently recorded by both reviewers in separate databases and only compared at the end of the data extraction process to limit selection bias. In case of disagreements, the categorization created by K.S. was honored. Duplicates were removed, and any disparities were clarified.
The 5,990 patients had a CT abdomen report mentioning PI, of which 411 patients had IBD. After excluding those reports specifying an absence of PI, 11 confirmed cases of PI with IBD remained. The mean age was 63.4 Ā± 22 years (range, 19-96 years) of which 7 were females, 4 had UC, 6 had CD and 1 had indeterminate colitis (Table 1). No patient had a prior CT scan demonstrating PI during the study period. PI was limited to the small bowel in 5 patients, to the right colon in 5, with one case of both small bowel and colonic PI. The only patient to undergo an endoscopic procedure during their admission was a 22-year-old female with UC. Incomplete colonoscopy 5 days following her CT revealed active disease with deep ulceration to the extent of examination in the sigmoid colon. Pre-admission medications included one case each of adalimumab, prednisone, azathioprine, and mesalamine.
During hospitalization 1 patient received adalimumab, 1 infliximab and corticosteroid, 2 corticosteroids, and 6 patients received broad spectrum antibiotics. Patients receiving biologic and/or corticosteroid were managed for IBD exacerbation. For those patients receiving antibiotics alone, there was either clinical uncertainty regarding IBD exacerbation or the primary management was directed towards suspected infection or sepsis. Only 1 patient, a 20-year-old male with CD, had no additional medical treatment during hospitalization. He continued on his pre-admission azathioprine, was given intravenous fluids and low residue diet and was discharged without complication after 6 days. Two patients, both with CD, had surgery. One patient needed emergent exploratory laparotomy for pneumoperitoneum and the other had ileocolic resection for stricture and obstruction.
There were 3 mortalities (27%) all with CD; 2 with isolated small bowel PI and one with both small bowel and colonic PI and all with evidence of portal and/or mesenteric venous gas. The first, a 69-year-old male was admitted for acute kidney failure and fevers prior to recognition of PI. His cause of death was attributed to worsening renal failure. The 2 other patients died from sepsis. One, a 76-year-old female died immediately following an exploratory laparotomy and resection of extensive small bowel necrosis. The other, a 96-year-old male expired during supportive comfort care, with elimination of blood draws and with an emphasis on pain management. All 3 had received antibiotics, without corticosteroid or biologic therapy. The remaining 8 patients made a full recovery and were discharged from the hospital.
Ten studies met inclusion criteria (Fig. 1) of which 9 articles primarily described patients with PI, including some with IBD, while 1 described the prevalence of PI from a population of CD patients (Table 2) [11-20]. There were no differences in data extraction noted between the 2 reviewers. In total, 58 IBD patients with PI were identified. IBD disease type was defined in 7 of the 10 studies including 21 with CD and 13 with UC. A mean age of 33.5 years was available in the article reporting on PI within a CD population and in another article, a single patient was 49 years [11,12]. Data on medical management was available on 6 patients across 2 studies [11,12]. One patient was treated through observation and had an uneventful recovery [12]. There were no outcomes data on the remaining 5 patients [11]. Utilization of surgery was noted in 2 articles, identifying 2 patients who underwent surgery [11,13]. No outcomes were reported. Nine articles reported on mortality [12-20]. Pooling the data results yielded an overall mortality rate of 22% for patients with PI, but there was no specific data available on mortality in the subset of patients with both PI and IBD. The limited extractable data specific to IBD patients in the studies precluded meta-analysis.
In our case series we have observed that a CT finding of PI in a hospitalized IBD patient is a rare event, with mortality limited to those CD patients with small bowel PI and CT evidence of portal/mesenteric venous gas. As only a minority of patients identified were treated with corticosteroids and/or biologic therapy suggesting an IBD exacerbation, it remains unclear whether a finding of PI is related to IBD severity in this population. As expected for a rarely observed entity, the systematic review confirmed that there is minimal data available on the prevalence, management and outcomes of PI in the setting of IBD. Only one of the studies, by John et al. [11] provided a prevalence of PI in a group of patients with CD, finding PI in 6 out of 50 patients (12%) undergoing CT abdomen. We suspect that this high prevalence may reflect the selection of a sicker group of patients for CT evaluation, as access to CT was more limited during the study time period. Management, both medical and surgical, was also rarely reported across the included studies. Though the pooled results of the systematic review noted a similar mortality rate of 22% among all PI patients, mortality among IBD patients was not specifically addressed and as such cannot be compared to our own centerās experience.
Limitations to our findings are those common among retrospective studies. Despite access to the admission medical record, and high reliability of tracking treatment during hospitalization, we cannot be sure of the accuracy of historical data such as medication use in the days and months prior to hospitalization. Also, though one might suspect that a population with worsening IBD would have undergone invasive testing such as a sigmoidoscopy or colonoscopy in the near term prior to admission, no such procedures were recorded. In such cases, it would be plausible that PI was not merely the result of worsening disease activity, but perhaps related to procedural trauma. Additionally, since no commonly employed objective clinical disease activity scores where obtained during the patientsā admission it remains unclear whether there was a relationship between IBD severity and the finding of PI. As such we cannot draw a direct link between IBD disease activity and outcomes in this group. Also, given the small number of cases, and the lack of established treatment protocols, little can be concluded regarding the relationship between treatment and outcomes. Additionally, though our methodology is dependent upon the radiologist to report PI when observed, itās unlikely that such a rare and significant finding would be left out of the final CT report. Conversely, important benefits to the methodology of patient identification are also worth mentioning. Rather than relying solely upon diagnosis coding to identify those with PI and IBD, our ability to first identify PI by a direct search of all CT abdomen reports decreases the possibility of missing cases of PI. Though the subsequent identification of IBD in this group involved cross referencing with IBD diagnosis codes, the final small number of cases identified allowed for direct chart review in these individuals to confirm their IBD diagnosis.
In conclusion, a systematic search of all CT scan reports from emergency room and inpatient encounters at 2 large tertiary care hospitals during the study period revealed only 11 cases of PI occurring in the setting of IBD. As demonstrated by the results of the systematic review, this appears to be the first reporting of its kind in a broad inpatient IBD population. Also, this would appear to be the first reporting outside of case reports describing in detail the disease distribution of PI with IBD and its associated outcomes. The small numbers unfortunately do not allow conclusions as to the best management practices, whether medical or surgical, when PI is found to complicate IBD. Despite the limited numbers, the high mortality rate observed in those with PI small intestinal involvement, and portal/mesenteric venous gas appears to identify a high-risk patient appropriate for early intensive care unit care and surgical consultation. Conversely, those without these features appear to do well with supportive medical care alone. Further studies, both retrospective and prospective will be needed to define best practices for patient management of this rare but complicated disease presentation.
NOTES
TableĀ 1.
Patient Clinical Characteristics, Treatment, and Outcome
TableĀ 2.
Systematic Review Included Articles
| First author | Year | Type of paper | Total no. of patients discussed in article | No. of IBD&PI patients | No. of CD&PI patients | No. of UC&PI patients | Patients with HPVG, PI, and IBD | Surgery as treatment for IBD and PI patients | Conservative treatment for IBD and PI patients | Mortality rate reported of entire sample (%) | Mortality reported of patients with IBD+PI (%) | IBD and PI patients specific information |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ferrada [14] | 2017 | IBDpatients within PI population | 127 | 6 | 2 | 3 | Not recorded | Not recorded | Not recorded | 22 | Not recorded | Not recorded |
| Treyaud [15] | 2017 | IBD patients within PI population | 149 | >2 | >1 | >1 | Not recorded | Not recorded | Not recorded | 42 | Not recorded | Not recorded |
| Umapathi [16] | 2016 | IBD patients within PI population | 217 | 10 | Not recorded | Not record | Not recorded | Not recorded | Not recorded | 18 | Not recorded | Not recorded |
| Lee [17] | 2014 | IBD patients within PI population | 123 | 2 | Not record | Not record | Not recorded | Not recorded | Not recorded | 24 | Not recorded | Not recorded |
| DuBose [18] | 2013 | IBD patients within PI population | 500 | 27 | 10 | 8 | Not recorded | Not recorded | Not recorded | 17 | 0 | Not recorded |
| Lassandro [19] | 2010 | IBD patients within PI population | 102 | 1 | 1 | 0 | 0 | Not recorded | Not recorded | 30 | 0 | Not recorded |
| Wayne [12] | 2010 | IBD patients within PI population | 88 | 1 | 1 | 0 | 0 | Not recorded | 1 | 18 | 0 | 49-Year-old male presenting with PI and history of CD being managed on steroids. |
| Greenstein [13] | 2007 | IBD patients within PI population | 40 | 2 | 0 | 1 | Not recorded | >1 | Not recorded | 20 | Not recorded | Not recorded |
| John [11] | 1992 | PI patients within IBD population | 50 | 6 | 6 | 0 | Not recorded | 1 | 5 | Not recorded | Not recorded | Of the patients with PI, 2 were male and 4 were female. They had a mean age of 33.5 years old. At the time of presentation, their current medications were sulfasalazine (2), steroids (6), azathioprine (1). |
| Knechtle [20] | 1990 | IBD patients within PI population | 27 | 1 | 0 | 0 | Not recorded | Not recorded | Not recorded | 33 | 0 | Not recorded |
REFERENCES
1. Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 2004;126:1504-1517.


2. Malik TA. Inflammatory bowel disease: historical perspective, epidemiology, and risk factors. Surg Clin North Am 2015;95:1105-1122.


4. Friedman S, Blumberg RS. Inflammatory bowel disease. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson JL, Loscalzo J. eds. Harrisonās principles of internal medicine. New York: McGraw-Hill, 2014:351-353.
5. Wedlake L, Slack N, Andreyev HJ, Whelan K. Fiber in the treatment and maintenance of inflammatory bowel disease: a systematic review of randomized controlled trials. Inflamm Bowel Dis 2014;20:576-586.


6. Wald A. Pneumatosis coli (pneumatosis cystoides intestinalis). In: Feldman M, Friedman LS, Brandt LJ. eds. Sleisenger and Fordtranās gastrointestinal and liver disease. Philadelphia: Saunders Elsevier, 2016:2306-2308.
7. Galandiuk S, Fazio VW, Petras RE. Pneumatosis cystoides intestinalis in Crohnās disease: report of two cases. Dis Colon Rectum 1985;28:951-956.


8. Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life-threatening causes. AJR Am J Roentgenol 2007;188:1604-1613.


9. Yale CE, Balish E, Wu JP. The bacterial etiology of pneumatosis cystoides intestinalis. Arch Surg 1974;109:89-94.


10. Maizlin ZV, Vos PM. Do we really need to thank the Beatles for the financing of the development of the computed tomography scanner? J Comput Assist Tomogr 2012;36:161-164.


11. John A, Dickey K, Fenwick J, Sussman B, Beeken W. Pneumatosis intestinalis in patients with Crohnās disease. Dig Dis Sci 1992;37:813-817.



12. Wayne E, Ough M, Wu A, et al. Management algorithm for pneumatosis intestinalis and portal venous gas: treatment and outcome of 88 consecutive cases. J Gastrointest Surg 2010;14:437-448.



13. Greenstein AJ, Nguyen SQ, Berlin A, et al. Pneumatosis intestinalis in adults: management, surgical indications, and risk factors for mortality. J Gastrointest Surg 2007;11:1268-1274.



14. Ferrada P, Callcut R, Bauza G, et al. Pneumatosis Intestinalis Predictive Evaluation Study: a multicenter epidemiologic study of the American Association for the Surgery of Trauma. J Trauma Acute Care Surg 2017;82:451-460.


15. Treyaud MO, Duran R, Zins M, Knebel JF, Meuli RA, Schmidt S. Clinical significance of pneumatosis intestinalis: correlation of MDCT-findings with treatment and outcome. Eur Radiol 2017;27:70-79.



16. Umapathi BA, Friel CM, Stukenborg GJ, Hedrick TL. Estimating the risk of bowel ischemia requiring surgery in patients with tomographic evidence of pneumatosis intestinalis. Am J Surg 2016;212:762-768.


17. Lee HS, Cho YW, Kim KJ, Lee JS, Lee SS, Yang SK. A simple score for predicting mortality in patients with pneumatosis intestinalis. Eur J Radiol 2014;83:639-645.


18. DuBose JJ, Lissauer M, Maung AA, et al. Pneumatosis Intestinalis Predictive Evaluation Study (PIPES): a multicenter epidemiologic study of the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 2013;75:15-23.