Novel biomarkers for the diagnosis and prognosis of colorectal cancer
Article information
Abstract
Colorectal cancer (CRC) is among the most common malignancies and remains a major cause of cancer-related death worldwide. Despite recent advances in surgical and multimodal therapies, the overall survival of advanced CRC patients remains very low. Cancer progression, including invasion and metastasis, is a major cause of death among CRC patients. The underlying mechanisms of action resulting in cancer progression are beginning to unravel. The reported molecular and biochemical mechanisms that might contribute to the phenotypic changes in favor of carcinogenesis include apoptosis inhibition, enhanced tumor cell proliferation, increased invasiveness, cell adhesion perturbations, angiogenesis promotion, and immune surveillance inhibition. These events may contribute to the development and progression of cancer. A biomarker is a molecule that can be detected in tissue, blood, or stool samples to allow the identification of pathological conditions such as cancer. Thus, it would be beneficial to identify reliable and practical molecular biomarkers that aid in the diagnostic and therapeutic processes of CRC. Recent research has targeted the development of biomarkers that aid in the early diagnosis and prognostic stratification of CRC. Despite that, the identification of diagnostic, prognostic, and/or predictive biomarkers remains challenging, and previously identified biomarkers might be insufficient to be clinically applicable or offer high patient acceptability. Here, we discuss recent advances in the development of molecular biomarkers for their potential usefulness in early and less-invasive diagnosis, treatment, and follow-up of CRC.
INTRODUCTION
Colorectal cancer (CRC) is a leading cause of cancer-associated morbidity and mortality worldwide [1]. Despite evidence of a 5-year survival rate of 90% when CRC is diagnosed at an early stage, less than 40% of cases are diagnosed when the cancer is still localized [1]. Most CRC cases develop from precursor lesions defined as adenomatous polyps [2]. Currently available screening programs for CRC are administered using guaiac-based fecal occult blood tests, fecal immunohistochemical test, flexible sigmoidoscopy, or colonoscopy. Colonoscopic polypectomy is an effective screening and prevention modality for detecting and treating precancerous and early cancerous lesions to decrease CRC incidence and mortality rates [3].
Rapid advances in our understanding of the molecular and biological characteristics of CRC have provided useful knowledge about its pathogenesis [1,2]. It has become possible to the develop biomarkers that help with the identification of patient responses with respect to cancer diagnosis, management, and surveillance [4,5]. The identification of biomarkers that can aid CRC early detection or monitoring would enable the development of personalized medicine and improve survival rates.
An ideal CRC biomarker should be easily and quantitatively measured, highly specific and sensitive, reliable, and reproducible. It should also be able to differentiate between different risk-based populations and select patients who require a second-line test (endoscopic and radiologic investigations). Ideally, these aims can be achieved with a noninvasive and inexpensive method using easily available biological samples such as urine, breath, serum, or feces [6-9]. Despite advances made in recent years, no single test is currently able to diagnose and monitor the posttreatment course of CRC patients. Here, we review the current status of newer diagnostic, prognostic, and predictive biomarkers in CRC and provide insights for their implementation in clinical management.
DIAGNOSTIC BIOMARKERS (Table 1)
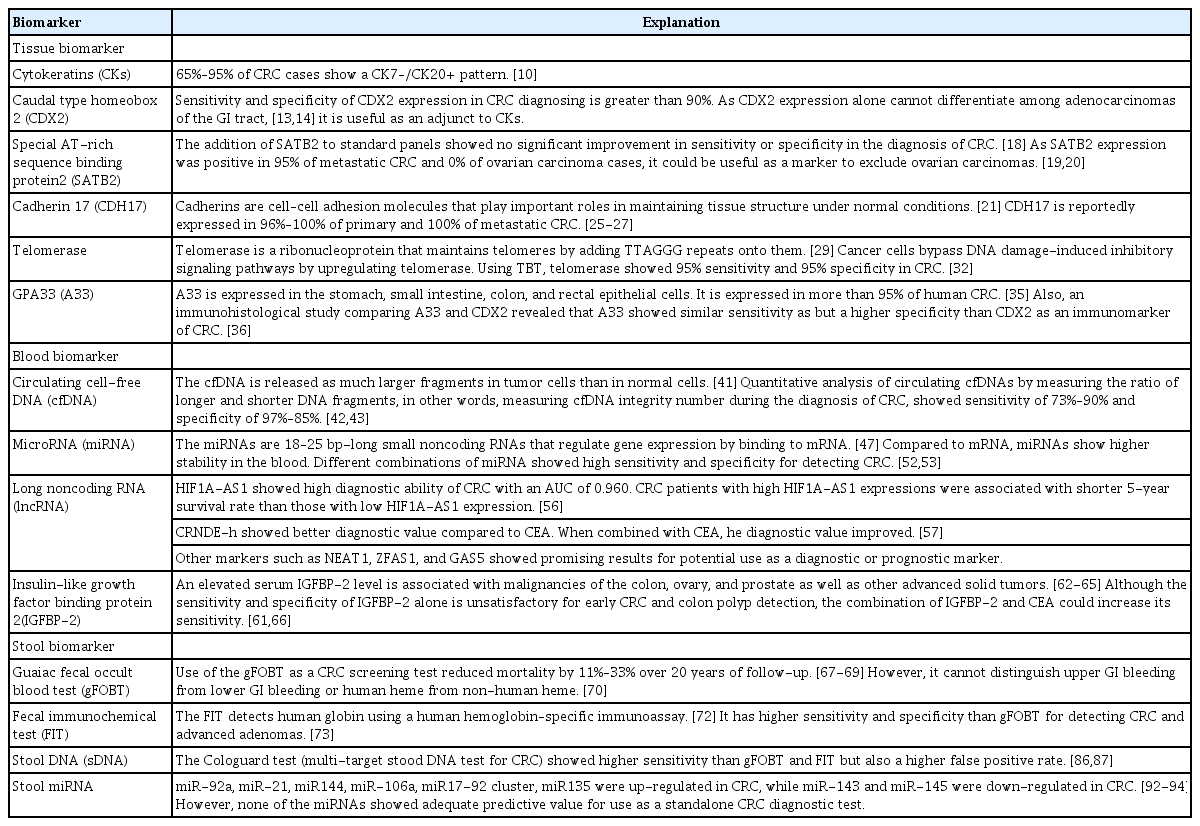
1. Tissue Biomarkers
1) Cytokeratins
Cytokeratins (CKs) are keratin proteins found in the intracytoplasmic cytoskeleton of epithelial tissue. To differentiate metastatic CRC from other tumors, tissues can be stained for CK7 and CK20. CRC specimens usually stain positive for CK20 and negative for CK7 [10]. CK20 is selectively present in the normal gland cells of the colonic mucosa and Merkel cells. In contrast, CK7 is not detected in the colonic mucosa. CK7 is expressed in bladder and female genital tract epithelia, mesothelium, and normal lung [11]. CK staining patterns are among the most helpful procedures for identifying metastatic adenocarcinoma of unknown primary origin. Detecting the CK7–/CK20+ pattern is a typical method for metastatic CRC diagnosis [12]. A reported 65% to 95% of CRC cases show a CK7–/CK20+ pattern [10].
2) Caudal type homeobox 2
Caudal type homeobox 2 (CDX2) codes for a homeobox protein that is critically involved in the regulation of normal cell differentiation in the GI tract and tumor suppression in the colon. Werling et al. [13] revealed that the loss of CDX2 expression might result in CRC. They also found that immunohistochemical detection of CDX2 protein expression could be used to identify CRC from other adenocarcinomas of the GI tract. According to the study findings, high CDX2 expression levels were found in CRC, while intermediate CDX2 expression levels were found in other adenocarcinomas of the GI tract. Other than CK pattern, assessing CDX2 expression is also a very sensitive and specific manner of identifying CRC. Many studies have evaluated CDX2 expression in CRC, and its sensitivity and specificity are reportedly greater than 90% [13,14]. However, many studies found that CDX2 is also expressed in other adenocarcinomas of the GI tract such as adenocarcinoma of the stomach (33%–70%). CDX2 expression alone cannot differentiate among adenocarcinomas of the GI tract [13,14]. Therefore, it is very useful when used as an adjunct to CK staining, especially in patients with a CK7+/CK20+ or CK7–/CK20– profile.
3) Special AT-rich sequence binding protein 2
Special AT-rich sequence binding protein 2 (SATB2) is part of the matrix attachment region-binding transcription factors family [15]. Although the exact role of SATB2 in the GI tract is unknown, Magnusson et al. [16] found that SATB2 is highly expressed in the epithelium of the lower GI tract including the appendix, colon, and rectum. The authors checked the expression profile of SATB2 in 216 cancer samples and found that the majority of CRC samples, including that of poorly differentiated CRC, showed strong SATB2 expression. SATB2 used as a single marker showed positivity in 87.8% (943/1,074) of CRC cases [16,17]. Other carcinomas from the ovary and lung rarely stained positive for SATB2 expression ( < 7%) [17]. Many studies have evaluated the benefit of the addition of SATB2 to the standard panels of CK7, CK20, and CDX2. Dragomir et al. [18] reported that the addition of SATB2 to standard panels showed no significant improvement in sensitivity or specificity in the diagnosis of CRC. In contrast, 2 recent studies reported that SATB2 could be a specific marker for differentiating metastatic CRC from primary ovarian carcinomas. Before the identification of SATB2, CDX2 was the most specific marker available. However, CDX2 tested positive in 18% of ovarian carcinomas, whereas SATB2 was expressed in 95% of metastatic CRC and 0% of ovarian carcinomas [19,20]. Therefore, SATB2 could be used as a marker to exclude ovarian carcinomas.
4) Cadherin 17
Cadherins are cell–cell adhesion molecules that play important roles in maintaining tissue structure under normal conditions [21]. Molecular defects in cadherin expression are associated with many human diseases, including carcinomas [22]. Cadherin 17 (CDH17), a member of the cadherin superfamily, was first identified in the rat liver and intestine [23]. Later on, 3 immunohistochemical studies consisting of a large number of human tissues found that CDH17 was expressed on the surface epithelium of the duodenum, ileum, appendix, and colon [24-26]. Many recent studies have indicated that CDH17 could be a good immunohistochemical marker for the diagnosis of adenocarcinomas of the GI tract. CDH17 is reportedly expressed in 96% to 100% of primary and 100% of metastatic CRC [25-27]. CDH17 is also expressed in other GI tumors such as gastric, pancreatic, and biliary cancer but it is rarely found outside the GI tract [28]. Although CDH17 is transcriptionally regulated by CDX2, some studies indicated that CDH17 is more sensitive and specific than CDX2 for the identification of CRC [27,28].
5) Telomerase
Telomerase is a ribonucleoprotein that maintains telomeres by adding TTAGGG repeats onto telomeres that are located at the ends of chromosomes. Telomerase uses intrinsic RNA as a template for reverse transcription [29]. In normal cells, telomeres shorten with each cell division. When telomeres become critically shortened, a signal of DNA damage is induced that results in replicative senescence [30]. Cancer cells bypass DNA damage-induced inhibitory signaling pathways by upregulating telomerase. Telomerase is found in 85% to 90% of all malignant tumors [31]. Using the novel assay Telomerase Biosensor Technology (TBT; Sienna Cancer Diagnostics, Melbourne, Australia), telomerase has become a new diagnostic biomarker in CRC. According to the study, it is predicted to show 95% specificity and 95% sensitivity in melanoma, bladder cancer, and CRC [32].
6) GPA33
The GPA33 (A33) gene codes for membranous protein A33, a membrane-bound glycoprotein with a homolog in the immunoglobulin superfamily [33]. Although the function of A33 is not yet understood, it might be associated with immunological processes, proliferation, and colonic mucosal repair as reported by an animal study [34]. Immunohistochemical studies have found that A33 is expressed by epithelial cells in the stomach, small intestine, colon, and rectum. It is expressed in more than 95% of human CRC cases, especially in well-differentiated tumors, indicating that it could be a potential target for CRC treatment [35]. An immunohistological study comparing A33 and CDX2 revealed that A33 showed sensitivity similar to that of CDX2 but specificity higher than that of CDX2 as an immunomarker of CRC [36].
2. Blood Biomarkers
1) Chromosomal instability
Chromosomal instability (CIN) is defined as gain or loss of whole or large portions of chromosomes leading to karyotypic variability, resulting in sub-chromosomal genomic amplifications, changes in chromosome numbers, and a high loss of heterozygosity (LOH) rate. CIN is the most common form of genetic instability observed in CRC (65%–70%) [37]. Mutations in tumor suppressor genes (TSGs) and oncogenes such as APC, CTNNB1, KRAS, PIK3CA, and TP53, and LOH of chromosome 18q were the key events that lead to the development of CIN CRC [38-40].
2) Circulating cell-free DNA
Circulating cell-free DNA (cfDNA) is a type of cell-free nucleic acid derived from normal and tumor cells that enters into the bloodstream by apoptosis or necrosis [41]. The lengths of cfDNA strands differ among processes by which they are made. In healthy individuals, cfDNA is released from apoptotic cells, and the DNA fragments are about 180 bp long. However, in tumor cells, cfDNA is released as much larger fragments by necrosis [41]. Therefore, the quantitative analysis of circulating cfDNAs by measuring the ratio of longer and shorter DNA fragments, in other words, measuring cfDNA integrity number during the diagnosis of CRC, showed some promising results. Hao et al. [42] reported that cfDNA integrity number measured by qualitative PCR method showed a sensitivity of 73.08% and specificity of 97.27%. Later, El-Gayar et al. [43] reported that cfDNA integrity number measured by RT-PCR reaction showed sensitivity of 90% and specificity of 85%. The concentrations of cfDNA differed between healthy and CRC patients. CRC patients showed a five times higher concentration of serum cfDNA and 25 to 50 times higher concentrations of plasma cfDNA than healthy controls [43-45]. Wang et al. [46] performed a systematic review and meta-analysis of circulating cfDNA as a diagnostic marker for CRC. Fourteen studies were analyzed which included 1,258 CRC patients and 803 healthy controls. Quantitative analysis of circulating cfDNA for the diagnosis of CRC showed 73.5% sensitivity and 91.8% specificity, suggesting that it had acceptable specificity for the diagnosis of CRC. The integrity index showed better diagnostic accuracy for CRC than did absolute DNA concentration.
3) MicroRNA
MicroRNAs (miRNAs) are 18–25 bp-long small noncoding RNAs that regulate gene expression by binding to mRNA. The miRNAs are reportedly associated with many cancers including CRC by acting as oncogenes or TSGs [47]. Compared to mRNA, miRNAs show higher stability in the blood since they avoid degradation by endogenous RNase and are resistant to extreme pH changes. For these reasons, miRNA are promising noninvasive biomarkers in cancer [48]. Studies have assessed their diagnostic ability for CRC in single or panels of miRNA.
Many studies reported that miR-21 could be a promising diagnostic marker for CRC. One study reported that miR-21 showed 90% specificity and sensitivity for CRC detection [49,50]. Another study reported that the combination of miR-141 and CEA improved CRC detection accuracy. High levels of miR141 were associated with poor survival, indicating that it may be used as a prognostic marker [51].
Other studies assessing different combinations of miRNAs showed better results. A panel of mi-24, mi-320a, and mi-423-5q showed 92.8% sensitivity and 70.8% specificity for detecting CRC [52]. Another study reported that a panel of 6 serum miRNA (miR-21, let-7g, miR-31, miR-92a, miR-181b, and miR203) showed 93% sensitivity and 91% specificity for detecting CRC, comparable to those of conventional tumor markers CEA and CA19-9 (carbohydrate antigen 19-9) [53].
4) Long noncoding RNAs
Long noncoding RNAs (lncRNAs), which consist of more than 200 nucleotides that cannot translate to protein, are implicated in various biological processes such as epigenetic regulation, immune responses, differentiation, and chromosome dynamics [54]. At this point, more than 150 human disease are reportedly associated with lncRNAs, such as colon cancer, breast cancer, leukemia, and psoriasis [55]. Studies have assessed their diagnostic ability for CRC using single or panel lncRNAs.
Serum hypoxia-inducible factor (HIF) 1alpha-antisense RNA 1 (HIF1A-AS1) was significantly elevated in 151 CRC patients versus that in 160 healthy controls and showed a high diagnostic ability for CRC with an area under the curve (AUC) of 0.960 (95% CI, 0.940–0.980; P< 0.001). CRC patients with high HIF1A-AS1 expression were associated with a shorter 5-year survival rate than those with low expression, indicating that HIF1A-AS1 could be used as a CRC diagnostic and prognostic biomarker [56].
Colorectal neoplasia differentially expressed-h (CRNDE-h), a splice variant of CRNDE, was shown to distinguish CRC patients from healthy individuals. Serum CRNDE-h levels were significantly elevated in CRC patients compared to those in patients with benign disease or healthy controls. Compared to conventional tumor marker CEA, the diagnostic value of CRNDE-h was better that the AUC value for distinguishing CRC patients from healthy controls was 0.892 and 0.688, respectively. AUC value improved to 0.913 when CRNDE-h levels were combined with CEA. Also, the elevated CRNDE-h level was significantly associated with lymph node involvement and distant metastases, and patients with an elevated CRNDEh level had poor overall survival (OS) rates [57].
Other lncRNAs such as NEAT1 [58], ZNFX1 antisense RNA1 (ZFAS1) [59], and GAS5 [60] showed some promising results of their potential use as CRC diagnostic or prognostic biomarkers.
5) Insulin-like growth factor binding protein 2
Insulin-like growth factor binding protein 2 (IGFBP-2) is a binding protein that modulates the interaction between insulin-like growth factor (IGF) ligands and IGF-1 receptors [61]. Although the physiological role of IGFBP-2 is not clearly understood, several studies have reported that serum IGFBP-2 level elevations are associated with malignancies of the colon, ovary, and prostate as well as other advanced solid tumors [62-65]. Liou et al. [66] reported that elevated serum and plasma IGFBP-2 levels could distinguish patients with CRC or colon polyps from healthy controls. Although the sensitivity and specificity of IGFBP-2 alone is unsatisfactory for early CRC and colon polyp detection, its combination of IGFBP-2 with other biomarkers such as CEA could increase the sensitivity. Also, higher plasma IGFBP-2 levels were associated with larger tumor size and worse OS rates, indicating that IGFBP-2 might serve as a diagnostic and prognostic biomarker for CRC [61].
3. Stool Biomarkers
1) Fecal occult blood and immunochemical test
The guaiac fecal occult blood test (gFOBT) and fecal immunochemical test (FIT) are widely used non-invasive techniques for screening for CRC. According to randomized control clinical trials, gFOBT as a CRC screening test reduced mortality by 11%–33% over 20 years of follow-up [67-69]. However, its use as a biomarker has many limitations: It cannot distinguish bleeding between the upper and lower GI tract; cannot distinguish human heme from non-human heme; and is easily affected by drugs or diet [70]. Therefore, it shows only 30% to 40% sensitivity for detecting cancerous and precancerous lesions [71]. These limitations of gFOBT have led to the development of FIT, which detects human globin using a human hemoglobin–specific immunoassay. It can detect both the presence and the quantity of fecal hemoglobin. Therefore, the cutoff level for this test can be selected, which might be advantageous [72]. Compared to gFOBT, FIT has many advantages: it has higher sensitivity and specificity for CRC and advanced adenomas (AAs) [73]; it requires only 1 stool sample (vs. 3); it is more cost-effective; and it provides more quality-adjusted life years than gFOBT [74]. However, the FIT also has limitations. In detecting adenomas > 1 cm in diameter, FIT showed only 20% to30% sensitivity [75]. Moreover, the occult blood test can detect left-sided lesions in the colon much more than right-sided lesions [75,76]. This is an important limitation since the incidence of right-sided CRC has been increasing over the past 2 decades [77]. Therefore, neither gFOBT nor FIT can stand alone as a diagnostic tool.
2) Other stool-based tests
Stool-based assays are considered the most successful type for many reasons. According to direct histological observations, CRC and polyps exfoliate many neoplastic cells and their debris into the mucocellular layer of the colonic lumen [72]. The detectable molecular changes that are caused by CRC cells are reportedly present in the stool earlier than in the blood [78]. The actual unfolded surface area of the epithelial monolayer of cancers and polyps could be 200 times larger than that predicted by gross findings [79]. However, despite abundant cellular exfoliation from large surface areas and neoplastic cells in the mucocellular areas, exfoliated colonocytes rarely survive if they are shed in the right colon due to intra-luminal lysis [80]. Hence, after cell lysis, the detection of components of the exfoliated cells, such as DNA, miRNA, and proteins, could be useful.
3) Stool DNA
Less than 0.01% of the total DNA in the stool is human DNA, while the other 99.99% is derived from intestinal bacteria or the diet. Therefore, detecting methylated or mutated human DNA in the stool is an important technique for diagnosing CRC [81]. Several panels of methylated genes within stool DNA (sDNA), such as APC, ATM, BMP3, CDH1, CDKN2A, CDH13, CRBP1, CXCL21, ESR1, GATA4, GSTP1, HLTF, ID4, IRF8, ITGA4, KRAS, MINT1, MINT31, MLH1, MGMT, NDRG4, RASSF2A, SFRP2, TFPI2, VIM, and WIF1, have been analyzed for the diagnosis of CRC [82-85]. To date, the United States Food and Drug Administration has approved only the Cologuard test (multitarget stool DNA test for CRC) for CRC screening. Compared to the gFOBT, the Cologuard test shows better sensitivity (13% vs. 52%) for detecting CRC. However, no improvement was seen in detecting large ( > 1 cm) adenomas (10.3% vs. 10.7%) [86]. Similar results were observed when the sDNA test was compared to the FIT: the former showed higher sensitivity but a higher false positive rate as well [87]. To improve the early detection rate of CRC and AAs, some studies have combined the sDNA test and FIT. The majority of studies reported that the sensitivity for detecting CRC and AAs was higher with the sDNA test plus the FIT compared to the FIT alone. However, since the false positivity rate was higher, the demand for colonoscopy was more than twice that of the sDNA test plus FIT compared to the FIT alone. Also, the specificity for detecting CRC was lower for the sDNA test plus the FIT compared to the FIT alone [88-90].
4) Stool miRNA
Since the environment of the GI tract is much more complicated than that of the blood, marker stability within it is a major concern. Many studies have shown that, unlike rapid degradation of mRNA and protein, miRNA transcripts were more stable in various conditions [47,91]. Although there are limited data about miRNA in the stool compared to that in the blood, dysregulation of miRNA expression was found in the stool of CRC patients; miR-92a, miR-21, miR144, miR-106a, miR17-92 cluster, and miR135 were up-regulated in CRC; and miR-143 and miR-145 were down-regulated in CRC [92-94]. However, none of the miRNAs showed adequate predictive value for use as a CRC diagnostic test alone, and future studies are needed to improve the diagnostic value of miRNA by combining several miRNAs [92].
PROGNOSTIC BIOMARKERS (Table 2)
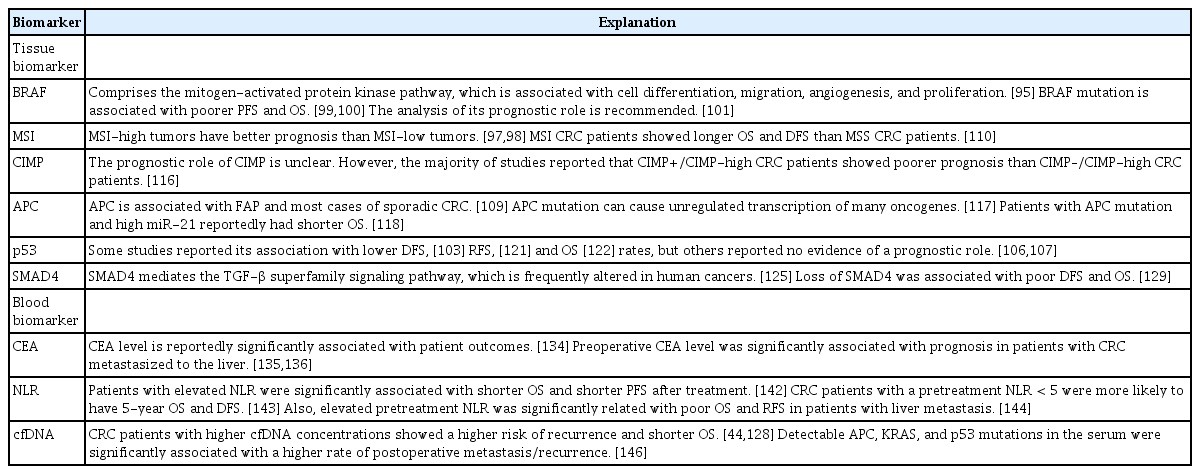
1. Tissue Biomarkers
1) BRAF
The mitogen-activated protein kinase pathway, which consists of RAS-RAF-MEK-ERK, is associated with cell differentiation, migration, angiogenesis, and proliferation. The dysregulation of this pathway leads to carcinogenesis [95]. Approximately 8% of advanced CRC and 14% of localized stage II and III CRC cases have BRAF-activating mutations [96,97]. According to the metaanalysis of Li and Li [98], the BRAF mutation was significantly associated with a tumor location in the proximal colon, poor differentiation, tumor size, and female sex. Advanced CRC patients with the BRAF mutation showed poorer progressionfree survival (PFS) and OS rates and lower response rates to anti-epidermal growth factor receptor (EGFR) therapy than those without BRAF mutations. Patients with localized stage II and III CRC with BRAF mutations also showed poorer OS rates [99,100]. Supported by these results, in 2017, the American Society of Clinical Oncology (ASCO) published the guideline for the use of molecular biomarkers for CRC. BRAF p.V600 accounts for more than 90% of BRAF mutations, and is recommended to be analyzed for its prognostic value [101].
2) Microsatellite instability
Microsatellites are repeating DNA sequences of 1–6 bp that can be found in coding and noncoding regions. The mismatch repair (MMR) system fixes DNA errors that occur during replication. Microsatellite instability (MSI) results from inactivation of the MMR genes through sporadic MLH1 promoter hypermethylation (80% of MSI CRC cases) or germline mutations in MMR genes such as MLH1, MSH2, MSH6, or PMS2 (20% of MSI CRC cases) [102,103]. The presence of deficient MMR leads to the accumulation of somatic mutations and induces genomic instability, causing cancer-associated alterations [104]. Lynch syndrome, also called hereditary non-polyposis colon cancer, is caused by germline mutations in MMR genes that lead to MSI [105]. MSI is also associated with sporadic CRC. In sporadic MSI CRC, hypermethylation of the MLH1 promoter region causes MLH1 silencing [106]. MMR status testing is recommended for patients with CRC for prognostic stratification [107]. There are 5 commonly used microsatellite markers, including 2 mononucleotide repeats (BAT26 and BAT25) and 3 dinucleotide repeats (D2S123, D5S346, and D17S250). If a marker shows greater than 30%–40% instability, it is identified as MSIhigh [108]. MSI-high tumors are more likely to be poorly differentiated, contain mucin, and possess subepithelial lymphoid aggregates and intraepithelial lymphocytes. Although the cause is unknown, MSI-high tumors have better prognosis than MSIlow tumors, possibly due to these immune responses [108,109]. Guastadisegni et al. [110] reviewed 13 studies to evaluate the prognostic value of MSI in CRC patients and reported that MSI CRC patients showed longer OS and disease-free survival (DFS) than microsatellite stable CRC patients.
3) Cytosine preceding guanine island methylator phenotype
Cytosine preceding guanine (CpG) islands are regions that are common in promoter sites rich in CpG dinucleotides. Abnormal DNA methylation has been observed in almost all CRCs. CpG island methylator phenotype (CIMP) CRC (10%–20%) have extremely high proportions of aberrantly methylated CpG loci [111]. Hypermethylation in CpG islands silences genetic activity and result in dysregulated gene expression [112]. CIMP is often defined as hypermethylation of at least 3 loci in a selected panel of 5 genes (hMLH1, p16, MINT1, MINT3, and MINT31) that are associated with CpG islands [113]. Since CIMP was a recently identified phenotype, methylated loci that are used to define CIMP have not yet been established [114,115]. This might be the reason why the prognostic role of CIMP remains unclear. However, the majority of studies reported that CIMP+/CIMPhigh CRC patients showed poorer prognosis than CIMP–/CIMPhigh CRC patients [116].
4) APC
APC gene mutation is associated with familial adenomatous polyposis and most sporadic CRC cases [109]. APC has an important role in Wnt signaling, which is associated with cytoskeletal integrity, cellular proliferation, motility, and apoptosis by β-catenin regulation. APC mutation can increase β-catenin levels, leading to increased c-myc expression, which is associated with cell proliferation. Therefore, APC mutation can cause the unregulated transcription of many oncogenes [117]. It was recently reported that in advanced-stage CRC, patients with APC mutation and high miR-21 had shorter OS, indicating that APC mutation could be a measure to predict the clinical outcomes of CRC [118].
5) p53
CRC (50%–70%) have mutations in the TSG p53 [119]. When DNA is damaged, p53 causes cell cycle arrest to repair the mutations; if the mutations cannot be repaired, apoptosis is induced [109]. Many studies have examined p53 mutations and their prognostic value in CRC patients. However, the results are contradictory. Some studies reported that p53 mutation/overexpression is associated with lower DFS [120], relapse-free survival (RFS) [121], and OS [122] rates. Other studies reported that there is no evidence that p53 status has prognostic value [123,124].
6) SMAD4
Mutations in the TSG SMAD4, located on chromosome 18q21, leads to the loss of SMAD4 protein expression. SMAD4 mediates the transforming growth factor-β superfamily signaling pathway, which is frequently altered in human cancers [125]. It is associated with cell proliferation, differentiation, apoptosis, and cell migration [126]. A reported 30% to 40% of CRC cases show SMAD4 mutations [127,128]. Voorneveld et al. [129] performed a meta-analysis to clarify the prognostic value of SMAD4 in CRC patients. According to the study findings, loss of SMAD4 was associated with poor DFS and OS.
2. Blood Biomarkers
1) CEA levels
CEA is the only marker that has been recommended by the ASCO 2006 update of recommendations for the management of CRC patients. CEA testing every 3 months post-surgery is recommended for patients with stage II or III CRC [130]. Renehan et al. [131] reviewed 5 trials of patient follow-up after curative resection of CRC. The authors reported that intensive follow-up consisting of CEA testing every 3–6 months and CT every 3–12 months significantly reduced mortality. According to another meta-analysis done by Rosen et al. [132], the intensive follow-up group showed a higher cumulative 5-year survival rate than the control group (72.1% vs. 63.7%).
Many studies have supported the prognostic value of preoperative CEA levels [133]. Park et al. [134] analyzed 2,230 CRC patients and found that CEA level was significantly associated with patient outcomes. According to 2 large-scale case studies, preoperative CEA level was significantly associated with prognosis in patients with CRC that metastasized to the liver [135,136].
2) Neutrophil-to-lymphocyte ratio
Lymphopenia is associated with impaired cell-mediated immunity, while neutrophilia is associated with systemic inflammation [137]. The neutrophil-to-lymphocyte ratio (NLR) was first studied as a marker for immune responses to various stressful conditions [138]. Other studies found potential for NLR as a prognostic marker for pancreatic cancer [139], gastric cancer [140], and hepatocellular carcinoma [141]. However, few systemic reviews and meta-analyses have examined the prognostic role of NLR in CRC. Li et al. [142] reported that patients with elevated NLR was significantly associated with shorter OS and PFS after treatment. Also, elevated NLR was significantly associated with elevated CEA level. Tsai et al. [143] reported similar results after analyzing 15 studies including 7,741 CRC patients. CRC patients with a NLR < 5 before treatment were more likely to have 5-year OS and DFS. Tang et al. [144] reviewed and analyzed a total of 1,685 CRC patients from 8 studies and reported that elevated pretreatment NLR was significantly related to poor OS and RFS in patients with liver metastasis.
3) Concentration of cfDNA
A higher cfDNA concentration is reportedly related to significantly shorter OS in CRC patients. Furthermore, CRC patients with higher cfDNA levels showed a higher risk of recurrence and shorter OS [44,145]. Similarly, Wang et al. [146] observed APC, KRAS, and p53 mutations in the serum and found that patients with detectable cfDNA showed significantly higher rates of postoperative metastasis/recurrence than those without detectable cfDNA. Another study reported that patients with the KRAS mutation in the plasma and tissue showed shorter OS, indicating that the KRAS mutation in the plasma could be a prognostic marker for a poor outcome [147].
PREDICTIVE BIOMARKERS (Table 3)
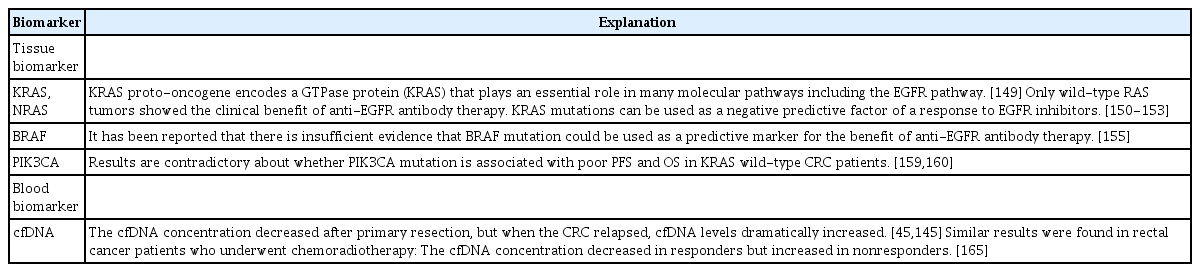
1. Tissue Biomarkers
1) KRAS and NRAS
It has been reported that more than 50% of CRC cases show KRAS, NRAS, and BRAF mutations [148]. The KRAS proto-oncogene encodes a GTPase protein (KRAS) that plays an essential role in many molecular pathways, including the EGFR pathway [149]. Only wild-type RAS tumors reportedly showed the clinical benefit of anti-EGFR antibody therapy such as cetuximab and panitumumab. As confirmed by both retrospective and prospective trials, KRAS mutations can be used as a negative predictive factor of a response to EGFR inhibitors [150-153]. In 2017, ASCO reviewed 74,546 patients in 311 primary studies that compared treatment outcomes of patients with activated RAS mutation to those of the non-mutated type and recommended RAS mutational testing in all patients being considered for anti-EGFR therapy [154].
2) BRAF
A meta-analysis performed by Rowland et al. [155] to evaluate the effect of BRAF mutation on anti-EGFR antibody therapy revealed that there was insufficient evidence that BRAF could be used as a predictive marker for anti-EGFR antibody therapy success. Although the hazard ratio for OS and PFS with anti-EGFR antibody therapy was higher in RAS wild-type/BRAFmutated tumors than in RAS wild-type/BRAF wild-type tumors (OS: 0.97; 95% CI, 0.67–1.41 vs. 0.81; 95% CI, 0.70–0.95 and PFS: 0.89; 95% CI, 0.61–1.21 vs. 0.62; 95% CI, 0.50–0.77, respectively); the results were not statistically significant (P= 0.43 and P= 0.07, respectively).
3) PIK3CA
PIK3CA encodes the p110α catalytic subunit of the class IA phosphatidylinositol 3-kinases (PI3Ks), which play a major role in the RAS-mediated pathway that leads to proliferation, transformation, and tumor progression [156]. PIK3CA mutation occurs 10% to 18% of CRC patients, primarily in exons 9 and 20 [150].
Many studies have reported that PIK3CA mutation might help predict a lack of benefit from anti-EGFR therapy in colon cancer, but the results were inconclusive [157,158]. Huang et al. [159] conducted a meta-analysis of 11 studies with 864 KRAS-wildtype metastatic CRC patients treated with anti-EGFR monoclonal antibodies. According to the study findings, patients with PIK3CA mutation showed a reduced response rate and poor PFS and OS in KRAS wild-type metastatic CRC patients. In particular, the PIK3CA mutation in exon 20 was significantly associated with a lack of response. On the other hand, Karapetis et al. [160] reported that PIK3CA mutation status cannot be used as a predictive marker for a benefit from anti-EGFR monoclonal antibodies. Their results showed that PIK3CA mutation was not associated with lower OS or PFS from cetuximab therapy in KRAS wild-type CRC patients. Many studies have reported the protective effect of aspirin in CRC [161,162]. A recent study reported that PIK3CA mutation could be a predictive marker for adjuvant aspirin therapy in CRC patients. Liao et al. [163] studied 964 patients with CRC and found out that PIK3CA-mutated CRC patients showed longer cancer-specific survival and OS rates compared to PIK3CA wild-type CRC patients.
2. Blood Biomarkers
1) Cell-free DNA
Studies have found that the cfDNA concentration decreased after primary resection, but upon CRC relapse, cfDNA levels dramatically increased [45,145]. Another study found that when circulating tumor DNA was detected after CRC surgery, it generally relapsed within 1 year [164]. These results indicate that postoperative cfDNA measurement could predict and help detect recurrence earlier. Zitt et al. [165] evaluated the cfDNA concentration before and after chemoradiotherapy for rectal cancer. The authors found that cfDNA concentration decreased in responders, whereas it increased in non-responders. Agostini et al. [166] reported that post-chemoradiotherapy levels of the cfDNA integrity index was significantly lower in rectal cancer patients who responded to the therapy. Similar results were observed in other cancer studies. Patients who responded to the therapy showed decreased cfDNA, whereas those who did not respond to the therapy showed no change or increase in cfDNA [167].
CONCLUSIONS
CRC is a common malignancy that contributes significantly to cancer mortality rates. Survival outcomes of CRC vary between patients because of the complexity of colorectal carcinogenesis. Therefore, it would be beneficial to identify reliable and practical molecular biomarkers that help in the diagnostic and therapeutic process of CRC. Recent research has been targeted to identify sensitive and specific biomarkers for the diagnosis and treatment outcomes of CRC. Here we provided an overview of the newer diagnostic, prognostic, and predictive biomarkers of CRC. Future studies are required to develop accurate diagnostic, prognostic, and predictive CRC biomarkers that could be more clinically applicable and offer greater patient acceptability than conventional biomarkers.
Notes
FINANCIAL SUPPORT
The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION
Conceptualization, writing, editing: Joo YE. Conceptualization and writing: Oh HH. Approval of final manuscript: all authors.