INTRODUCTION
Ulcerative colitis (UC) is an idiopathic, nonspecific inflammatory disease that affects the colonic mucosa. The disease is chronic, with repeated episodes of relapse and remission, and the main symptoms are rectal bleeding (RB), diarrhea, and abdominal pain. UC is characterized by lesions that spread continuously from the rectum, progressing towards the ascending colon, which can extend throughout the entire colon. Depending on its extension, UC is classified as proctitis, leftsided colitis, or pancolitis. Topical therapy, consisting of enemas or suppositories, is used to control the distal lesions and is usually the first choice of clinicians based on European and American clinical practice guidelines [
1,
2].
Budesonide 2-mg foam (BF) in a single injection can be distributed from the rectum to the sigmoid colon [
3]. Although BF is a glucocorticoid with high receptor affinity [
4] and has potent anti-inflammatory action at the treatment site, it is quickly metabolized in the liver with low systemic exposure [
5]. Foam preparations are expected to reduce leakage, a problem with liquid enemas [
6], because foam has high retention within the rectum. Additionally, with this treatment method, BF can achieve a wider range of distribution than a suppository [
3]. Currently, BF is widely prescribed in Japan for UC; for non-severe cases, the recommended dosage is usually a single rectal administration of BF (containing 2 mg) given twice daily. It is recommended that patients are carefully monitored during BF use, and the necessity of continuing BF therapy for 6 weeks should be carefully considered.
Mucosal healing (MH) has been regarded as the therapeutic goal of UC treatment. Complete MH has been associated with reduced subsequent rates of relapse [
7,
8], hospitalization [
9], and surgery [
10]. Additionally, patients with a Mayo endoscopic subscore (MES) of 0, indicating complete MH, have a better prognosis than those with an MES of 1 [
11]. We recently conducted randomized clinical trials targeting an MES of 0 as an efficacy endpoint of BF treatment.
The superiority of BF twice a day for 6 weeks over placebo for attaining complete MH was confirmed in UC patients with active mucosal inflammation in phase II and phase III clinical trials in Japan [
12,
13]; however, few studies have investigated the predictive factors for MH in patients treated with BF. Furthermore, there is a concern that because topical therapy administration is more complicated than that of oral medication, patient adherence to topical therapy is poor [
14]. Although patients typically show improvement in RB symptoms early in treatment, Naganuma et al. [
13] found that patients may require 6 weeks of twice-daily treatment to achieve complete MH. For the purpose of improving patient adherence to BF treatment twice a day for 6 weeks, we conducted a
post hoc analysis of the pooled data from 2 clinical trials on BF conducted in Japan to explore the demographic and clinical factors that affect prognosis and to determine the predictors of the therapeutic effect of BF.
METHODS
1. Ethical Considerations
The phase II and III clinical trials [
12,
13] from which the data were obtained were conducted in compliance with the Declaration of Helsinki and Good Clinical Practice. The institutional review board of each center approved the protocol. All patients provided written informed consent.
2. Participants and Treatment Intervention
We conducted the present analysis using pooled data from phase II and phase III clinical trials (Japic CTI-132294 and Japic CTI-142704) evaluating the efficacy and safety of BF (2 mg/25 mL) in patients with UC in Japan. The details of the study designs, inclusion criteria, interventions, randomization, and blinding have been reported previously [
12,
13]. Briefly, patients were randomized at a ratio of 1:1:1 into 3 groups in the phase II clinical trial as follows: BF (once/day), BF (twice/day), or placebo foam. In the phase III clinical trial, patients were randomized at a ratio of 1:1 into 2 groups as follows: BF (twice/day) or placebo foam.
3. Analysis Procedures
A Modified Mayo Disease Activity Index (MMDAI) score was used to assess disease activity. The enrollment criteria were a stool frequency (SF) subscore of 0-2, RB subscore of 1-2, endoscopic subscores of 2 in the segment from the rectum to the sigmoid colon and 0-1 in the adoral segment beyond the sigmoid colon, and ≥ 12 weeks since UC diagnosis.
BF was administered for 6 weeks. Concomitant therapy with oral 5-aminosalicylic acid (5-ASA) agents, oral salazosulfapyridine agents, or probiotics in stable doses was permitted. The use of the following drugs and therapies was prohibited: 5-ASA rectal preparations or suppositories, salazosulfapyridine suppositories, corticosteroid preparations, cytapheresis, immunomodulators, antitumor necrosis factor antibody preparations, and surgical treatment for UC.
Because the approved BF regimen was twice-daily administration, patients administered BF once a day in the phase II study were excluded from the pooled population in this analysis.
4. Definition of Endpoints and Parameters
The efficacy endpoints were complete MH, clinical remission (CR), elimination of RB, and normalization of SF. CR was defined as an RB subscore of 0, endoscopic subscore of 0 or 1, and either an SF subscore of 0 or a decrease by at least 1 from baseline using the MMDAI subscore [
15]. Complete MH was defined as an MES of 0. The present active phase for each patient was defined as the period between the start of remission induction therapy in this active phase and study enrollment completion. Patients with a first attack were defined as any patient diagnosed with UC who were enrolled in the study during the first active phase of UC. A relapsing/remitting clinical course was defined as patients who had experienced CR of UC in the past and were enrolled in the study during a flare-up phase. These definitions were the same as those used in the 2 aforementioned clinical studies [
12,
13].
5. Outcomes
We evaluated the efficacy and safety of the approved dose of BF by using pooled data from the phase II and phase III clinical trials evaluating the efficacy and safety of BF for 6 weeks in UC patients.
The relationships between CR/complete MH at week 6 and the clinical characteristics of patients were analyzed. Additionally, we analyzed the relationships between CR/complete MH at week 6 and RB and SF subscores from baseline to week 6.
6. Statistical Analysis
Baseline demographics and clinical characteristics were summarized and compared between treatment groups using appropriate descriptive statistics and statistical tests. The number of patients who achieved the defined efficacy endpoints (complete MH, CR, and elimination of RB) and the achievement rates at week 6 were calculated, along with the 95% CI, by treatment group. The difference in achievement rates among the treatment groups was evaluated using the Fisher exact test, and a P-value was calculated.
The number of patients who achieved the defined efficacy endpoints, the number of patients who did not achieve the defined efficacy endpoints, and each of the achievement rates were calculated and classified by patient characteristics. Additionally, the proportion of patients who achieved each of the efficacy endpoints was evaluated using comparative tests, and each P-value was calculated. Statistical analyses were conducted at a significance level of 0.05 (two-sided).
To evaluate the factors affecting the achievement of efficacy endpoints, the number of patients who achieved each of the efficacy endpoints was counted and each achievement rate was calculated and classified by patient characteristics. Additionally, a logistic regression analysis was conducted with the efficacy endpoint as the dependent variable and patient background characteristics as the explanatory variable-age, sex, body weight, smoking habits, disease duration, first attack/relapse/remitting classification, duration of present active phase, extent of past lesions, MMDAI score, subscore for endoscopic, RB and SF, physician’s general evaluation score, previous medication for UC (i.e., oral 5-ASA preparation, 5-ASA enema/suppository, adrenocortical hormone, cytapheresis, immunomodulator, anti-TNFα antibody, other [excluding other investigational drugs], unknown or other investigational drugs), concomitant drugs such as oral 5-ASA formulation, and severity: total of each subscore (endoscopic, RB, and SF); an odds ratio was calculated along with the 95% CI and P-value. The results of the analyses are presented using the following 3 models: univariate (the explanatory variable is one item), multivariate first (the explanatory variables are all items with P< 0.10 in the univariate model), and multivariate final (the explanatory variables with P< 0.05 are selected using the step-down procedure). Additionally, an exact logistic regression was used to estimate regression parameters if the model was inappropriate when the data structure was near separation.
The number of patients who achieved complete MH was determined, and the achievement rate at week 6 was calculated using RB or SF subscores. The number of patients who achieved CR was determined, and the achievement rate at week 6 was calculated using RB or SF subscores. In addition, the Cochran-Armitage test was used to evaluate the relationship between complete MH or CR at week 6 and the RB or SF subscore for 6 weeks, and each P-value was calculated. Furthermore, proportional curves for patients with an RB or SF subscore of 0 after BF treatment were calculated with the last observation carried forward method to impute the data. The data (RB subscore, endoscopic subscore, and SF subscore) for patients who discontinued the study at less than 6 weeks of treatment were also imputed with the last observation carried forward method.
DISCUSSION
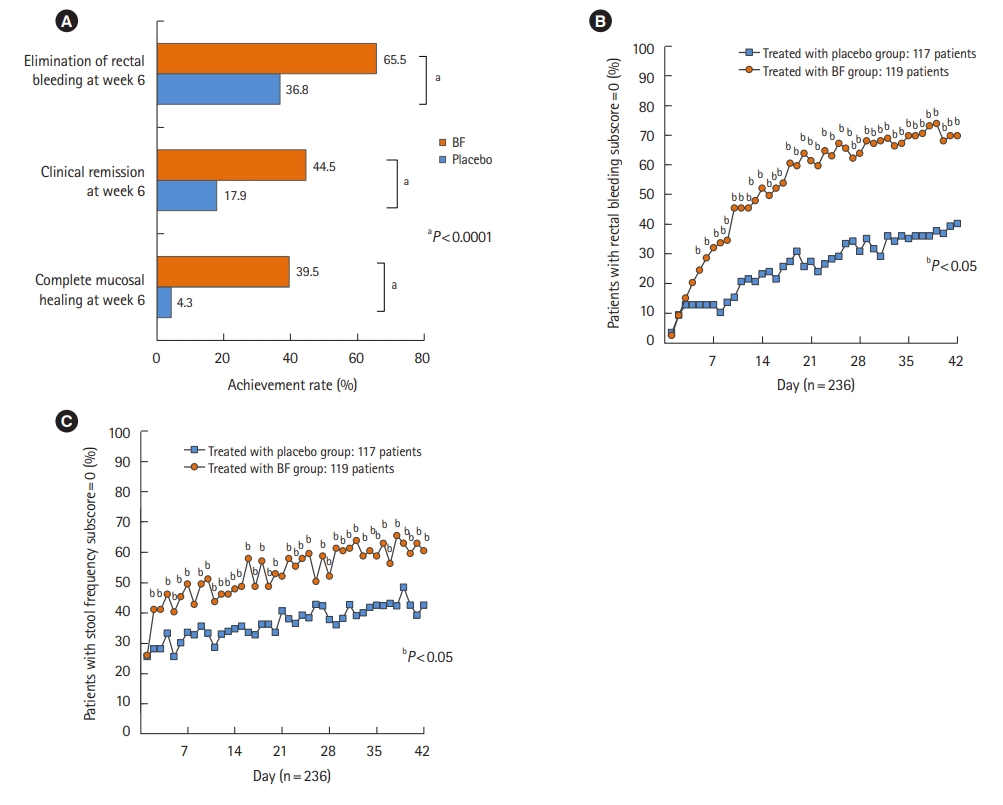
This was a post hoc analysis of pooled data from phase II and III clinical trials evaluating the efficacy and safety of BF treatment in UC patients. We conducted this analysis to identify the demographic and clinical factors affecting prognosis and analyzed the relationship between complete MH or CR and RB or SF subscores from baseline to week 6 to identify predictors of the therapeutic effect of BF. In this analysis, improvement of clinical symptoms (i.e., improvement in the RB or SF subscores) was clearly observed after 5 days (RB subscore) and after 2 days (SF subscore) of BF treatment. Based on this finding, it was suggested that topical BF might contribute to early symptom relief.
As a result of the multivariate analysis to identify the background patient characteristics that may affect treatment outcomes (efficacy endpoints), we found that complete MH was influenced by the baseline SF subscore and the use of a 5-ASA enema or suppository. Approximately 45% of patients had previously received a 5-ASA rectal enema or suppository. The multivariate analysis showed a statistically significant difference between patients previously treated with local 5-ASA treatment and those not previously treated. It has been reported that local 5-ASA treatment results in MH [
16], and patients who had a history of local 5-ASA treatment and were enrolled in the original clinical study were likely to have an insufficient MH response. Thus, it is possible that they had more severe disease characteristics than patients without a history of local 5-ASA treatment. In order to eliminate the effect of 5-ASA topical product use, specific exclusion criteria (i.e., patients using mesalazine preparations [enema or suppository] or salazosulfapyridine preparation [suppository] 1-week before the date of qualification were excluded from the study) were set at the time of the clinical trial to ensure a 1-week washout period. Therefore, the efficacy of BF was considered to have been adequately evaluated.
In the multivariate analysis, CR was influenced by sex, clinical course (first attack or relapsing/remitting), and baseline SF subscore. Male patients were more likely to achieve CR at week 6 than female patients. Other studies have shown differences in efficacy by sex, with women more likely to respond than men [
9] and a higher relapse rate in women than in men [
17]. The results of this analysis suggest that further investigations into sex differences are warranted.
Additionally, relapsing/remitting patients were more likely to achieve CR at week 6 than those with a first attack. In this analysis, however, 105 patients had relapsing/remitting disease while only 13 were having their first attack. Thus, it is difficult to draw any conclusions from these data. We presume that the moderate symptoms of patients with relapsing/remitting UC could be more easily controlled by the treatment and patients with refractory relapsing/remitting UC were not registered in this study. However, some patients with a first attack could have perceived their symptoms as mild to moderate but may have actually had more severe disease and, thus, it may have been more difficult for them to achieve CR by week 6. Additionally, both RB and SF subscores during treatment seemed to be predictive of achievement of CR at week 6. As we only examined a small number of cases, further research with larger sample sizes must be conducted to clarify this result.
A low baseline SF subscore before BF treatment was found to be a characteristic of patients who achieved complete MH. We consider that the retention time of the compound solution within the inflammatory lesion may be attributable to this effect. Further, our analyses suggest that a normal SF at the initiation of treatment might be the most relevant patient characteristic for choosing BF treatment because complete MH can be achieved under this condition. According to the study by Brunner et al. [
3], it takes 6 hours to reach the maximum drug distribution of BF. Thus, patients who had a high SF subscore and frequent defecations may not have retained the drug for a sufficient amount of time, which may have resulted in a decreased absorption volume of BF and a weakened treatment effect. This finding highlights the need to follow the recommendations on the timing of BF administration in relation to the patient’s bowel movement patterns, such as avoiding BF treatment immediately after eating when peristaltic movements tend to increase. In this
post hoc analysis, the achievement rates of complete MH or CR among patients with proctitis and other types of previous lesions were similar. These results are consistent with previously reported pharmacokinetic data [
3] from a study conducted in Austria that evaluated the diffusion of a single rectal administration of BF labeled with
99mTc. That study reported that BF reached the sigmoid colon in all 12 patients with mild-to-moderately active UC.
A recent study [
9] indicated that incomplete MH was a factor related to a nonbeneficial outcome (use of immunosuppressive agents, hospitalization, and colectomy) in patients who were treated with steroids. The BF dose of 2 mg/25 mL administered twice a day was approved because the achievement rate for complete MH was higher in the group receiving BF twice a day than in the group receiving BF once a day in the phase II study [
18]. Although topical treatment is effective for UC patients, it is often used only on demand because of patient unacceptance or inconvenience [
14].
We found that RB subscores after week 2 during BF treatment were associated with the achievement of complete MH at week 6 in this analysis. However, there was no significant trend at weeks 4 and 6, suggesting that SF subscore during treatment was less predictive of achieving complete MH at week 6 than RB subscore. Thus, SF subscores after week 4 during BF treatment did not necessarily correlate with achievement of complete MH at week 6. The SF subscores up to week 2 could be useful predictors of complete MH at week 6, but not from week 4 onwards. These results suggest that RB subscore after week 2 may be useful as a predictive factor of complete MH achievement at week 6. In some patients, RB did not resolve early in treatment, but MH was achieved at week 6. Therefore, it is also important to observe and examine the status of RB in each patient in order to decide whether to continue treatment. Conversely, the complete MH rate at week 6 was 30% (9/30) with an RB subscore of 1 at week 4, but 0% (0/8) with an RB subscore of 2 at week 4. This suggests the need to decide whether to continue treatment after week 4 for UC patients with an RB subscore of 2 or higher. However, because the number of patients in this analysis was small, it seemed important to decide upon treatment continuation by evaluating the symptoms of patients individually.
We conclude that it is important to monitor RB after initiation of BF treatment to achieve complete MH at week 6, and it is recommended to determine the adequacy of treatment continuation based on the RB subscores at weeks 2 and 4.
Aside from evaluating the SF subscore at baseline, RB subscore after week 2 is significantly associated with complete MH and thus may help predict the effect of BF treatment. Regarding the adherence to treatment, we consider that this could be improved once patients receive the appropriate instructions for administering the medication, and patient awareness is increased regarding the effects of continuing treatment twice daily up to week 6.
As this pooled analysis did not yield any notable safety findings, it is worth iterating the results of the 2 individual trials [
12,
13]. In one study [
12], the incidence of adverse effects in the placebo group was lower (29.6%) than that in the BF bid group (67.9%), while in the other study [
13], the incidence was similar in the placebo (40.3%) and BF (45.3%) groups. No serious treatment-related adverse effects or deaths were reported in either study. In both studies [
12,
13], glucocorticoid-related adverse events occurring only in the BF groups were decreased plasma concentrations of cortisol and adrenocorticotropin; however, the levels returned to normal after the treatment was terminated.
This analysis has some limitations, including the relatively low number of patients. Additionally, this was a post hoc analysis of pooled data from 2 existing clinical trials, and multiplicity was not considered. Additional studies are needed to assess the possibility of another relationship between MH or CR and changes in the baseline RB or SF subscores in clinical practice.
In conclusion, our analysis suggests 3 patient characteristics, namely, history of 5-ASA topical product use, normal SF at baseline, and elimination of RB after week 2 were found to be associated with complete MH at week 6 with twice-daily BF treatment. These characteristics could be useful predictors of BF treatment effect.