Use of Thiopurines in Inflammatory Bowel Disease: A Consensus Statement by the Korean Association for the Study of Intestinal Diseases (KASID)
Article information
Abstract
Background/Aims
For decades, thiopurines have been the mainstay of inflammatory bowel disease (IBD) treatment and will play an important role in the future. However, complex metabolism and various side effects limit the use of these potent drugs in clinical practice. The Korean Association for the Study of Intestinal Diseases developed a set of consensus statements with the aim of guiding clinicians on the appropriate use of thiopurines in the management of IBD.
Methods
Sixteen statements were initially drafted by five committee members. The quality of evidence and classification of recommendation were assessed according to the Grading of Recommendations Assessment, Development and Evaluation system. The statements were then circulated to IBD experts in Korea for review, feedback, and then finalized and accepted by voting at the consensus meeting.
Results
The consensus statements comprised four parts: (1) pre-treatment evaluation and management strategy, including value of thiopurine S-methyltransferase screening, dosing schedule, and novel biomarkers for predicting thiopurine-induced leukopenia; (2) treatment with thiopurines with regards to optimal duration of thiopurine treatment and long-term outcomes of combination therapy with anti-tumor necrosis factors; (3) safety of thiopurines, especially during pregnancy and lactation; and (4) monitoring side effects or efficacy of therapy using biomarkers.
Conclusions
Thiopurines are an effective treatment option for patients with IBD. Management decisions should be individualized according to the risk of relapse and adverse events.
INTRODUCTION
Since the 1980s, thiopurine agents such as azathioprine (AZA) and 6-mercaptopurine (6-MP) have been widely used for the treatment of IBD. It has been reported that thiopurines are effective at maintaining remission in steroid-dependent or steroid-refractory CD and UC and have steroid-sparing effect.123 They have also shown efficacy in preventing postoperative recurrence of CD.45 Although their clinical implications are limited, thiopurines remain a mainstay of treatment of patients with IBD in the biologic era. However, possible toxicities such as bone marrow suppression, hepatotoxicity, and increased risk of opportunistic infections and malignancies interfere with the use of these potent drugs in clinical practice.
At present, there exist several questions regarding the optimization of thiopurine therapy. To balance the efficacy with safety and tolerability, thiopurine S-methyltransferase (TPMT) pretreatment screening, prudent dose escalation, and careful monitoring using novel biomarkers have been suggested.678 However, most of the current guidelines for thiopurine use in IBD have been established based on western data. It has been widely accepted that notable differences in the context of epidemiology, genetics, and clinical characteristics exist between Western countries and East Asia, including Korea.910 Therefore, there is a definite need to establish management strategies that are more suitable for Korean patients.
For this reason, the Korean Association for the Study of Intestinal Diseases (KASID) developed a set of consensus statements on thiopurine use in IBD. The aim of these statements is to offer guidance to clinicians on the appropriate use of thiopurines in the management of IBD.
METHODS
The committee for the development of consensus statements comprised five members of KASID (working group). The committee conducted extensive literature review regarding thiopurine use in IBD and developed initial statements. Sixteen consensus statements were drafted after several revisions. The statements consisted of four parts: (1) pre-treatment evaluation and management strategy, (2) treatment with thiopurines, (3) safety of thiopurines, and (4) monitoring of side effects or efficacy of therapy.
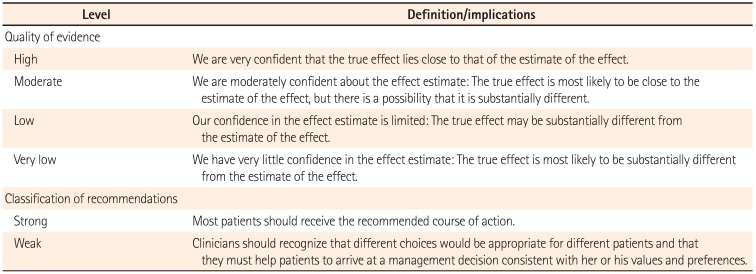
The quality of evidence and classification of recommendation was assessed according to the Grading of Recommendation Assessment, Development and Evaluation (GRADE) system.11 In this system, the quality of evidence of each statement was categorized as high, moderate, low, or very low. Evidence based on randomized controlled trials was initially classified as high-quality evidence, but the rating could be downgraded for several reasons including study limitations, inconsistency of results, indirectness of evidence, imprecision, and reporting bias. Although data from observational studies (e.g., cohort and case-control studies) were initially classified as low-quality evidence, the rating could be upgraded if the magnitude of the treatment effect is very large, if there is evidence of a dose-response relation, or if all plausible biases were found to decrease the magnitude of an apparent treatment effect.1112 Based on the GRADE system, the strength of recommendations was classified as strong or weak, determined by four key factors including the balance between the desirable and undesirable effects, quality of evidence, values and preferences, and costs (resource allocation).13 The definitions of the levels of evidence and recommendations are summarized in Table 1.
Prior to the consensus meeting, consensus statements were circulated to academic gastroenterologists with experience in managing patients with IBD and were revised in accordance with their comments and opinions regarding each statement.
The consensus meeting was held on April 18, 2015 in Seoul, Korea, wherein 38 IBD experts participated. During the meeting, the working group presented statements and all participants voted on their level of agreement regarding each statement. A consensus statement was accepted if at least 75% of participants voted 1 (strongly agree) or 2 (agree) on a scale of 1 to 5 (with 3, 4, and 5 indicating uncertain, disagree, and strongly disagree, respectively). If a statement was not accepted, the wording of the statement was discussed and revised, and then re-voting was conducted.
PRE-TREATMENT EVALUATION AND MANAGEMENT STRATEGY
Statement 1.
Thiopurine-Induced Leukopenia Is More Common in East Asians, Namely, Chinese, Japanese, and Koreans, Compared to Caucasians.
Quality of evidence: Moderate
Classification of recommendation: Strong
Level of agreement: Strongly agree 55%, Agree 40%, Uncertain 5%
Thiopurines have been widely used for the treatment of patients with IBD. Recommended dosages for the treatment of IBD are 2.0-2.5 mg/kg/day for AZA and 1.0-1.5 mg/kg/day for 6-MP.67 However, one of the major drawbacks of the use of thiopurines is the development of leukopenia (defined as white blood cell [WBC] count <3,000/mm3), occurring in up to 5% of Caucasian patients with IBD treated with these agents.141516 Interestingly, the occurrence of thiopurine-induced leukopenia is considerably high in Asians.171819202122 In a study by Lee et al.,17 thiopurine-induced leukopenia was observed in 116 (31.2%) of 372 Korean patients with CD at a median AZA dose of 1.34 mg/kg/day. In a study by Yang et al.,18 leukopenia was noted in 346 (35.4%) of 978 Korean patients with CD at a median AZA dose of 1.70 mg/kg/day. In another Korean multicenter study,19 leukopenia developed in 110 (39.6%) of 278 patients with IBD at a mean AZA dose of 1.80 mg/kg/day. In two Japanese IBD cohorts with wild-type TPMT,2021 leukopenia was observed in 18 (15.8%) of 114 patients and 7 (10.0%) of 70 patients, although the total daily dose of AZA was only 50 mg in the majority of patients. In a study by Fangbin et al.,22 leukopenia (WBC <3,500/mm3) was noted in 36 (18.1%) of 199 Chinese patients with IBD. Since all these East-Asian studies used doses lower than the recommended dosage of AZA, more frequent and severe leukopenia is expected with standard doses of thiopurines in this population.
Statement 2.
Assessment of the TPMT Genotype or Enzyme Activity Before Initiating Thiopurine Therapy Is of Limited Value in East-Asian Populations Compared to That in Caucasians.
Quality of evidence: Moderate
Classification of recommendation: Weak
Level of agreement: Strongly agree 29%, Agree 55%, Uncertain 16%
AZA and 6-MP are metabolized by TPMT. TPMT mutations that result in decreased enzymatic activity are associated with a greater risk of thiopurine-induced leukopenia.23242526 Therefore, in order to mitigate the risk of leukopenia with thiopurines, the US Food and Drug Administration recommends an assessment of TPMT status prior to initiating therapy.7 However, only about a quarter of IBD patients with thiopurine-induced leukopenia carry a TPMT mutation, thus raising questions on the utility of TPMT pre-testing.272829 Moreover, while the frequency of TPMT mutations is lower in Asians (1-3%)1819202122 than that in Caucasians (~10%),30 the occurrence of thiopurine-induced leukopenia in Asians is considerably high, as described in the Statement 1.171819202122 Consequently, TPMT mutations are present in only 0 to 5.6% of Asian patients with IBD who develop thiopurine-induced leukopenia.1819202122 In addition, homozygotes for TPMT mutant alleles are extremely rare (~0.01%) in the Japanese population 20 whereas their frequency is about 0.6% among Caucasians.31 Taken together, assessment of TPMT genotype/phenotype prior to thiopurine therapy is of limited value in East-Asian populations, when compared with Caucasians.
Statement 3.
Gradual Increase in the Dosage of Thiopurines Over Several Months Until the Target Dose Is Usually Preferable to Beginning the Medication at the Target Dose at the Onset, in East Asians.
Quality of evidence: Low
Classification of recommendation: Weak
Level of agreement: Strongly agree 58%, Agree 42%
The recommend target dose of AZA and 6-MP for IBD treatment is 2.0-2.5 mg/kg/day and 1-1.5 mg/kg/day, respectively, if the patients can tolerate the drugs.67 However, the best approach to attain the target dose has not been established yet. The traditional "dose escalating approach" is to initiate the therapy at 50 mg daily, then increase the dose by 25 mg every 1-2 weeks to a target dose along with monitoring for leukopenia and other potential adverse events.7 The main concern regarding this approach is under-dosing of patients with a suboptimal response rate. An alternate approach is to start immediately at the full standard dose based on TPMT activity. According to this approach, patients with normal TPMT activity receive the standard dose while the patients with intermediate TPMT activity receive 50% of the standard dose. However, patients with low TPMT activity (0.3% of general population) should not be treated with thiopurines.32 Interestingly, a questionnaire-based survey showed that 33% of US gastroenterologists initiated therapy at 50 mg of AZA, while 28% administered a full dose of AZA at 2.5 mg/kg/day, in clinical practice.33
Interestingly, the usual approach to prescribing thiopurines in East Asia is to start at a low dose and to gradually increase the dose.34353637 According to a survey on clinical practice patterns in the treatment of IBD in Korea, 80% of the responders initiated AZA at 50 mg/day, 68% increased the dose by 25 mg, and 56% increased the dose every 4 weeks.34 This gradual dose increment policy could be useful for reducing AZA-induced myelotoxicity.35 However, it may delay the time to clinical response. In China, a dose step-up strategy is typically carried out by gradually increasing the dose of AZA to the target dose under close monitoring of laboratory results and clinical response.36 In Japan, lower doses of AZA (50 mg/day or 0.6-1.2 mg/kg/day) are employed for the treatment of patients with IBD.2037 Unlike Western data, several studies have suggested that lower doses of AZA or 6-MP may be effective and safe for the treatment of patients with IBD in East Asia.17363738 However, these studies did not compare the efficacies of lower doses with those of standard doses; moreover, they did not analyze the 6-thioguanine nucleotide (6-TGN) levels. Nevertheless, considering higher incidences of myelotoxicity 171819202122 and limited value of the assessment of TPMT genotypes,1920212227282939 the dose escalating approach starting with low doses can be an effective and safe strategy in East Asians. However, there is currently no consensus on the rapidity of dosage increments.
Statement 4.
Determination of NUDT15 Genotypes Before Initiating Thiopurine Therapy May Identify Patients With a Predisposition to the Development of Thiopurine-Induced Early Leukopenia.
Quality of evidence: Moderate
Classification of recommendation: Strong
Level of agreement: Strongly agree 13%, Agree 76%, Uncertain 11%
As described in Statement 2, assessment of the TPMT genotype or enzyme activity may play a limited role in the prevention of thiopurine-induced leukopenia in East Asians. Recently, a non-synonymous single nucleotide polymorphism in NUDT15 variant was identified as a significant risk factor for thiopurine-induced early leukopenia in Korean patients with CD.18 The NUDT15 allele encoding p.Arg139Cys was found in 89.4% of the early leukopenia cases but was found in only 6.8% of the controls, suggesting that the presence of the NUDT15 allele had a sensitivity of 89.4% and specificity of 93.2% to early leucopenia. Although rare, this single nucleotide polymorphism was also associated with thiopurine-induced leukopenia in Caucasians.18 The frequency of NUDT15 risk allele is much higher in East Asians than that in Caucasians (10.4% in Koreans, 7% in Japanese, 13% in Chinese, and 2% in an admixed American population).18 The results of this study were reproduced by a study in children with acute lymphoblastic leukemia conducted in the United States.39 Therefore, pre-treatment determination of NUDT15 genotypes may help to identify patients susceptible to thiopurine-induced early leukopenia in diverse populations, especially in East Asians.
TREATMENT
Statement 5.
There Is Not Enough Evidence to Recommend Thiopurine Monotherapy for Induction of Remission in Active CD or UC.
Quality of evidence: Moderate
Classification of recommendation: Weak
Level of agreement: Strongly agree 42%, Agree 53%, Uncertain 5%
The data for thiopurines for remission induction in active UC is limited.4041 In a meta-analysis of 4 randomized controlled trials (n=89) evaluating the efficacy of AZA/6-MP for the induction of clinical remission of UC, the mean efficacy of AZA was not superior to placebo or 5-aminosalicylate (75% vs. 64%, respectively; OR, 1.59; 95% CI, 0.59-4.29; P=0.13).41 Thiopurines may be effective for inducing remission in active CD as compared to placebo (OR, 2.43; 95% CI, 1.62-3.64).42 However, a recent Cochrane meta-analysis including 11 randomized trials reported that thiopurines offered no significant advantage over placebo for induction of clinical remission in active CD (48% vs. 37%, respectively; RR, 1.23; 95% CI, 0.97-1.55).43 Usually, thiopurines have been limited in the use of remission induction in active CD or UC because of the delay in the onset of their action.44 Thiopurines may take 3-6 months to achieve full clinical efficacy.45 Therefore, in patients with active CD or UC requiring rapid symptom relief, concomitant therapy with systemic corticosteroids and thiopurines is a more reasonable approach. However, the addition of thiopurines to corticosteroids makes no benefit in terms of remission induction.44 Thiopurines have the benefit in corticosteroid sparing effect for active CD with a pooled OR of 3.69 (95% CI, 2.12-6.42).42
Statement 6.
Thiopurines Are Effective for the Maintenance of Remission in Patients With CD and UC.
Quality of evidence: High
Classification of recommendation: Strong
Level of agreement: Strongly agree 66%, Agree 32%, Uncertain 2%
Thiopurines are effective for maintenance of remission in both UC and CD.23 AZA appears to be more effective than placebo for the maintenance of remission in UC.3 According to a meta-analysis of 4 studies including 232 patients with UC, 44% (51/115) of patients administered AZA failed to maintain remission compared to 65% (76/117) of placebo-administered patients (RR, 0.68; 95% CI, 0.54-0.86).3 AZA or 6-MP may be effective as maintenance therapy in patients with UC who have failed to respond or cannot tolerate 5- aminosalicylate and in patients who require repeated courses of corticosteroids. In case of patients with CD, AZA or 6-MP also had a positive effect in maintaining remission.124647 A meta-analysis of 8 studies including 503 patients with CD revealed that the overall remission rate was 71% (147/208; 95% CI, 64-77%) for AZA treatment compared to that for placebo (55% [141/255]; 95% CI, 49-61%).2
AZA (1.0-2.5 mg/kg/day) is effective in reducing the risk of disease recurrence over a 6-month to 2-year period. Higher doses of AZA (2.5 mg/kg/day) are more effective than the lower doses (1.0 or 2.0 mg/kg/day) in preventing disease recurrence. In addition, AZA has corticosteroid-sparing effects that reduce corticosteroid-related side effects. Previously, a Korean study also reported that complete withdrawal of corticosteroids was achieved in 70.9% of patients with CD receiving treatment with AZA.17 AZA or 6-MP may also benefit patients dependent on corticosteroids or in cases where corticosteroid treatment has failed. Among Korean patients with steroid-dependent UC, the 3-year success rate was higher in the AZA therapy group (71.2%) than that in the AZA intolerance group (25.0%).38 However, withdrawals due to adverse events were more commonly observed in patients treated with AZA (Peto OR, 3.74; 95% CI, 1.48-9.45) compared to placebo.2
Statement 7.
Optimal Duration of Treatment With Thiopurines Is Uncertain. Prolonged or Indefinite Use of Thiopurines May Be Considered to Maintain Remission. The Risk and Benefits of Continuing Thiopurine Therapy Should Be Balanced and Discussed With Individual Patients.
Quality of evidence: Low
Classification of recommendation: Weak
Level of agreement: Strongly agree 68%, Agree 29%, Uncertain 3%
Conclusive evidence suggests that thiopurines are effective for maintenance of remission in both CD and UC.23 Well-tolerated and effective therapy is generally continued to maintain the remission of IBD. However, the optimal duration of thiopurine use has not yet been determined. There is limited data regarding the factors predicting responses to AZA and the uncertainty regarding the optimal duration of treatment.48 In patients with CD, several studies showed relapse rates of 14-41% at 1 year after cessation of thiopurines with a cumulative increase with time.495051 Furthermore, patients with CD who discontinued AZA after more than 3 years of efficacious treatment had a higher probability of relapse than those who continued therapy.52 A recent meta-analysis showed that there is a clear benefit of continuing AZA/6-MP therapy for at least 18 months to maintain remission in patients with CD.53 In patients with UC, relapse rates after cessation of AZA treatment were reported to be 35-77% and 65-75% at 1 and 5 years, respectively.165455 There was no difference in the relapse rates between CD and UC. The duration of AZA treatment did not affect the relapse rates after cessation of treatment (P=0.68).16 According to the European Crohn's and Colitis Organisation (ECCO) guidelines,56 for patients with CD who have been treated with thiopurines as part of maintenance therapy, discontinuation may be considered after 4 years of remission.
To summarize these data, thiopurines should be used for at least 18 months after achieving remission and could be continued for over 4 years. Therefore, considering the high cumulative relapse rates after cessation of thiopurine treatment, risk stratification is an important issue for patients with IBD receiving thiopurines. Meanwhile, frequent adverse events sometimes led to discontinuation of the thiopurine agents. Most frequent adverse events were nausea, hepatotoxicity, myelotoxicity, and pancreatitis. Overall, around 10-28% of patients reported side effects and of which 50-80% discontinued the thiopurines as a result.57 In addition to risk factors for disease relapse, adverse events with long-term use such as serious infections and malignancies must be taken into account. Thus, benefits and risks of continuing thiopurines should be considered and discussed with each patient individually.
Statement 8.
The Combination of Anti-Tumor Necrosis Factor (TNF)-α Agents and Thiopurines Is More Effective Than That of Monotherapy With Anti-TNF-α Agents* or Thiopurines† Alone in Inducing Remission in Patients With Moderate to Severe CD.
*Anti-TNF-α Agents
Quality of evidence: Moderate
Classification of recommendation: Weak
Level of agreement: Strongly agree 26%, Agree 58%, Uncertain 16%
†Thiopurines
Quality of evidence: High
Classification of recommendation: Strong
Level of agreement: Strongly agree 70%, Agree 24%, Uncertain 6%
Several studies have reported the effectiveness of combination of anti-TNF agents with thiopurines in case of rheumatic diseases and IBD. Thiopurines improved the pharmacokinetics of anti-TNF agents and decreased the formation of anti-drug antibodies.58 In the SONIC (The study of Biologic and Immunomodulator Naive Patients in Crohn's disease) trial that included immunosuppressant-naive CD patients, the corticosteroid-free remission rates and mucosal healing rates were higher in those treated with a combination of infliximab plus AZA than those treated with infliximab or AZA alone.59 The corticosteroid-free remission rates at week 26 were 57% in those receiving combination therapy, 44% in those receiving infliximab alone, and 30% in patients receiving AZA alone. At week 50, the corticosteroid-free remission rates were 72%, 61%, and 55%. Additionally, mucosal healing rates were evaluated at week 26 and they were 44%, 30%, and 16.5%, respectively.59 In the GETAID (Groupe d'Etude Therap-utique des Affections Inflammatoires du tube Digestif) study, infliximab plus AZA/6-MP was found to be more effective than AZA/6-MP alone for the induction of remission in patients with corticosteroid-dependent CD.60
In case of adalimumab, a recent meta-analysis study demonstrated that adalimumab monotherapy was inferior when compared to combination therapy with adalimumab plus thiopurines (OR, 0.78; P=0.02) for induction of remission in patients with CD. However, the rate of remission at 1 year and the need for dose escalation was similar in both groups.61 Therefore in patients with moderate to severe CD, especially in case of immunosuppressant-naive patients with CD, a combination therapy with anti-TNF and thiopurines seems to be preferred mode of treatment in order to allow the patients to achieve the highest rate of rapid disease control and limited tissue damage. However, the benefit of combination therapy in immunosuppressant treatment-failed patients remains unclear.
Statement 9.
The Combination of Anti-TNF-α Agents and Thiopurines Is More Effective Than That of Monotherapy With Anti-TNF-α Agents* or Thiopurines† Alone in Inducing Remission in Patients With Moderate to Severe UC.
*Anti-TNF-α Agents
Quality of evidence: Moderate
Classification of recommendation: Weak
Level of agreement: Strongly agree 18%, Agree 66%, Uncertain 16%
†Thiopurines
Quality of evidence: High
Classification of recommendation: Strong
Level of agreement: Strongly agree 55%, Agree 42%, Disagree 3%
The main question that remains to be answered in this clinical situation is whether combination therapy with infliximab and thiopurines is clearly superior to monotherapy with infliximab or thiopurines alone, in patients with UC. Recently, the UC SUCCESS (Efficacy and Safety of Infliximab and Azathioprine Monotherapy or in Combination in Moderate to Severe UC) trial was performed to provide a suitable explanation for the above question.62 This study was a randomized, double-blind clinical trial for evaluating the efficacy and safety of 16 weeks of infliximab plus AZA, infliximab or AZA monotherapy in patients with moderate to severe UC.62 At week 16, a greater proportion of patients in the infliximab plus AZA achieved corticosteroid-free remission (39.7%) compared to 22.1% receiving infliximab alone (P=0.017) and 23.7% receiving AZA alone (P=0.032). Mucosal healing at week 16 occurred in 62.8% of patients receiving infliximab plus AZA, compared to 54.6% receiving infliximab alone (P=0.295) and 36.8% receiving AZA alone (P=0.001). Adverse events that required discontinuation of therapy were higher in the AZA alone (8%) than in the infliximab alone (3%) or the infliximab plus AZA group (4%). Although there are no high quality reports regarding the superiority of combination therapy except for the UC SUCCESS trial, infliximab-based combination therapy can be more therapeutically useful in patients with moderate to severe UC who previously do not appear to respond adequately to corticosteroid therapy. The combination of adalimumab and immunomodulator appeared mildly superior to adalimumab monotherapy for the induction of remission in CD.61 However, it is still unclear in case of UC.
Statement 10.
Selection Between Anti-TNF-α Monotherapy and Combination Therapy With an Immunomodulator May Be Individualized Based on the Risk of Relapse and Adverse Events. Currently, No Recommendation Can Be Given for the Duration of Combination Therapy.
Quality of evidence: Low
Classification of recommendation: No
Level of agreement: Strongly agree 32%, Agree 65%, Uncertain 3%
As described in Statements 8 and 9, combination therapy with infliximab and thiopurines is more effective than monotherapy with either agent alone in inducing corticosteroid-free clinical remission and mucosal healing in patients with CD and UC who are naive to either agent.5962 Meanwhile, the clinical benefits of continuing immunomodulators in patients with IBD who are refractory to these drugs when starting anti-TNF therapy are debatable. However, combination therapy with anti-TNF agents and immunomodulators may increase the risk of serious infections and malignancies. In a study with longitudinal cohort of 8,581 CD patients,63 monotherapy with steroids, immunomodulators, or anti-TNF agents was associated with an increased risk of tuberculosis, candidiasis, herpes zoster, and sepsis as compared to patients not receiving these medications. The combination of two or three medications further increased the risk of these infections. In contrast, according to the TREAT (Crohn's Therapy, Resource, Evaluation and Assessment Tool) registry,64 CD severity and use of prednisone or narcotic analgesics showed higher risks of serious infection, although an increased risk was also observed with infliximab. Moreover, treatment with immunomodulators including thiopurines and methotrexate was not a significant predictor of serious infection, and combination therapy with infliximab and immunomodulators did not increase the risk of infection, compared with infliximab monotherapy. Therefore, relationship between combination therapy with infliximab and immunomodulators and development of serious infections remains unclear. The risk of neoplasm in IBD patients receiving anti-TNF therapy is controversial.6566 The combination of anti-TNF agents and immunomodulators is associated with an increased risk of malignancies such as lymphoma and non-melanoma skin cancer.676869 The risks of these cancers are largely driven by the use of concomitant immunomodulators, particularly thiopurines.70
In order to decide between the combination therapy versus monotherapy approach, we should weigh the increased risk of complications induced by combination therapy against the increased risk of relapse observed with monotherapy. According to a decision analytic model, the benefits of combination therapy with infliximab and AZA would outweigh the risks in CD patients who are naive to either agent, unless serious infections occurred in 20% or more of the population or lymphoma in 3.9% or more.71 Old age (>65 years) is associated with a greater risk of serious infections and lymphoproliferative disorder.7273 Hepatosplenic T-cell lymphoma usually occurs in young men (<35 years) receiving thiopurine therapy for more than 2 years.74 Factors associated with the risk of relapse after cessation of concomitant immunomodulators include increased inflammatory markers, evidence of mucosal activity on endoscopy, short duration in remission before stopping therapy, and undetectable trough levels of anti-TNF.70 Therefore, withdrawal of concomitant immunomodulators may be considered in patients in deep remission for a considerable period and with detectable anti-TNF trough levels, especially in elderly patients or young male patients. Currently, there is no consensus on the duration of combination therapy with an anti-TNF agent and an immunomodulator.
SAFETY
Statement 11.
Use of Thiopurines Is Associated With an Increased Risk of Lymphoma and Non-Melanoma Skin Cancers. However, the Absolute Risks of These Malignancies Remain Low. These Risks Should Be Weighed Against the Substantial Benefits of Thiopurine Therapy.
Quality of evidence: Moderate
Classification of recommendation: Strong
Level of agreement: Strongly agree 51%, Agree 49%
According to a recent meta-analysis by Kotlyar et al.,75 the overall standardized incidence ratio (SIR) for lymphoma was 4.92 (95% CI, 3.10-7.78) in patients with IBD exposed to thiopurines: 2.80 (95% CI, 1.82-4.32) in 8 population studies and 9.24 (95% CI, 4.69-18.2) in 10 referral studies. In 8 population studies, an increased risk was noted among current users (SIR, 5.71; 95% CI, 3.72-10.1) but not among former users (SIR, 1.42; 95% CI, 0.86-2.34).75 Young male patients (<30 years) had the highest SIR, and aged patients (>50 years) showed the highest absolute risk.75 According to a recent meta-analysis involving 60,351 IBD patients, the pooled adjusted hazards ratio of developing non-melanoma skin cancers after exposure to thiopurines was 2.28 (95 % CI, 1.50-3.45).76 However, the results of this meta-analysis must be interpreted carefully owing to a marked heterogeneity between studies. There is not enough evidence to suggest that the modestly increased risk of lymphoma and non-melanoma skin cancers outweighs the benefit of thiopurines in IBD.6776
Statement 12.
Use of Thiopurines Is Not Associated With an Increased Risk of Postoperative Complications.
Quality of evidence: Low
Classification of recommendation: Weak
Level of agreement: Strongly agree 30%, Agree 67%, Uncertain 3%
Postoperative complications in IBD include abdominal sepsis, wound problem, anastomotic leakage, early reoperation as well as complicated systemic diseases leading to increased morbidity and mortality. In case of CD, a study by Colombel et al.77 reported that early postoperative complications did not increase in patients with CD treated perioperatively with immunosuppressive agents. However, in a study by Myrelid et al.,78 preoperative thiopurine therapy was associated with postoperative intra-abdominal septic complications in case of abdominal surgery for patients with CD. As for UC, in a study by Schaufler et al.,79 preoperative exposure to thiopurines was not associated with increased postoperative complications in a cohort undergoing colectomy for UC or IBD-U. Recently, a meta-analysis involving 21 studies with 6,899 IBD patients evaluated whether the preoperative use of immunosuppressive agents was associated with increased postoperative complications in patients with IBD.80 Of these 21 studies, 8 studies with 1,674 patients reported the impact of preoperative thiopurines on postoperative outcomes. The pooled risk ratio estimates for total complications and infectious postoperative complications were 0.97 (95% CI, 0.69-1.36) and 1.23 (95% CI, 0.66-2.29), thus suggesting no association between preoperative use of thiopurines and postoperative complications.80 However, most studies were retrospectively designed and there were large variations in the patient populations and outcome definitions.80
Statement 13.
Thiopurines Are Considered to Be Safe and Well Tolerated During Pregnancy. Discontinuing Thiopurine Therapy During Pregnancy May Precipitate a Flare Resulting in Adverse Neonatal Outcomes.
Quality of evidence: Moderate
Classification of recommendation: Weak
Level of agreement: Strongly agree 16%, Agree 71%, Uncertain 13%
The peak incidence of IBD occurs in women of childbearing age. Management of patients with IBD during pregnancy requires a challenging balance between optimal disease control and drug safety considerations. Fears about the potential harm of IBD medications to the developing fetus are very common. In a survey conducted in Australia, 36.1% of participants believed that any form of IBD medication was harmful to the unborn fetus.81 However, discontinuation of therapy may result in disease relapse during pregnancy, which carries a greater risk of adverse fetal outcomes (a higher rate of fetal loss, preterm birth, and low birth weight), according to a number of reports.828384858687
Conflicting data exist regarding the association between thiopurine use for IBD treatment in pregnancy and adverse pregnancy outcomes. A few studies reported an increased risk of fetal loss, preterm delivery, low birth weight, and congenital abnormalities when thiopurines were used during pregnancy.888990 However, these outcomes might have been caused by the underlying disease rather than thiopurines. A majority of recently conducted controlled or cohort studies have not shown an increase in congenital abnormalities.9192939495 According to a recent pharmacological study conducted on thiopurines in pregnant patients with IBD, although unborn children were exposed in utero to the pharmacologically active thiopurine metabolites 6-TGN, no major teratogenicity was observed.96 In another study, thiopurine use during pregnancy did not affect long-term development or immune function of children up to 6 years of age.97 Moreover, two recent meta-analyses did not demonstrate an increased risk of fetal loss, low birth weight, or congenital abnormalities despite intrauterine exposure to thiopurines.9899 However, an association with preterm birth was noted in one out of the two meta-analyses studies.98 In addition, 60% of newborns exposed to thiopurines in utero were anemic at birth.96
Existing guidelines suggest that thiopurines should not be discontinued during pregnancy.100101102103 In a worldwide survey among 175 IBD experts, 155 (89%) physicians replied that they would continue AZA therapy throughout pregnancy.4 The potential risks and benefits of thiopurine therapy should be discussed with a patient, ideally prior to conception.
Statement 14.
Breastfeeding Could Be Advised for Women on Thiopurine Maintenance Therapy Willing to Nurse Their Infants. The Risks and Benefits of Thiopurine Therapy Should Be Discussed With the Mother.
Quality of evidence: Moderate
Classification of recommendation: Weak
Level of agreement: Strongly agree 23%, Agree 64%, Uncertain 13%
Treatment with thiopurines is widely used to maintain remission in IBD. Female patients with IBD may require treatment with thiopurines during lactation to maintain remission. Breastfeeding by patients with IBD patients on thiopurines is probably safe because the presence of only trace amounts of AZA/6-MP metabolites has been noted in breast milk.
In a prospective study, the concentration of 6-MP was measured in 31 breast milk samples collected from 10 mothers receiving AZA.104 6-MP was detected in low concentrations (1.2 and 7.6 ng/mL, as compared to therapeutic immunomodulator level of 50 ng/mL in serum) in two samples, but was not detected in any of the other 29 samples.104 Additionally, AZA/6-MP metabolites were undetectable in the neonatal blood.97104 The majority of thiopurine metabolites are excreted in milk in the first 4 hours after intake of the drug.105 Hence, mothers should be counseled to use a breast pump 4 hours after medication intake to discard the first portion of milk produced after AZA intake in order to minimize the infant's exposure to the drug.
Breastfeeding by IBD patients undergoing thiopurine therapy did not affect long-term development or immune function. One retrospective study showed no differences in infection rate in children breastfed by mothers receiving thiopurines for IBD as compared to children breastfed by mothers without immunomodulator therapy.92 Another recent observational study showed no differences in any of the global medical and psychosocial health status in 9 breastfed infants (for median 7 months, range 3-13 months) and the formula-fed group.106 Thiopurines can be administered safely to women with IBD during lactation. Therefore, breastfeeding should be recommended after considering its beneficial therapeutic effects for the nursing mother with IBD. Appropriate counseling for lactation should be available for all lactating women with IBD in order to discuss the risks and benefits of thiopurine therapy.
MONITORING
Statement 15.
Close Follow-Up of Full Blood Counts Is Recommended in All Patients Taking Thiopurines. However, the Optimal Frequency of Measurement Has Not Been Evaluated Systematically.
Quality of evidence: Moderate
Classification of recommendation: Weak
Level of agreement: Strongly agree 28%, Agree 67%, Uncertain 5%
Thiopurines have been reported to increase the risk of myelotoxicity, particularly leukopenia. Myelotoxicity is one of the more common dose-dependent adverse effects with potentially serious clinical consequences. The use of complete blood counts (CBC) to monitor bone marrow suppression is of particular importance.107 Most cases of severe leukopenia occur abruptly early on in treatment.107 In a study by Lewis et al., the incidence of severe leukopenia (WBC <1,000/mm3) was highest in the first 8 weeks of thiopurine therapy with the median time from onset of therapy to first documentation of severe leukopenia being 24.5 days.108
There is currently no clear consensus on optimal frequency of blood monitoring.109 Several monitoring guidelines have been proposed.15107110111 A Western study stated that a CBC should be obtained weekly for one month, biweekly for the second month, monthly for four months and, bimonthly when the patient is stable after six months of treatment. Although further studies are needed in order to better define the optimal interval of CBC monitoring, it may be scheduled biweekly for the first two months, and then every 4-12 weeks thereafter, in general.
Statement 16.
The Dose Adjustment of Thiopurines Through Monitoring of 6-TGN and 6-Methylmercaptopurine (6-MMP) Levels Is Expected to Improve Efficacy and Reduce Side Effects in Patients Treated With Thiopurines.
Quality of evidence: Moderate
Classification of recommendation: Weak
Level of agreement: Strongly agree 18%, Agree 69%, Uncertain 13%
The variation between clinical efficacy and side-effect profile of thiopurines is may be attributed to individual differences in drug metabolism. 6-MP and its prodrug AZA are converted to 6-TGN, which is the main active metabolite responsible for therapeutic efficacy and myelotoxicity. On the other hand, metabolism of these products also yields 6-MMP, high levels of which are associated with increased hepatotoxicity.
These findings have led to strategies for advantageously shifting thiopurine metabolism toward optimal 6-TGN levels while decreasing 6-MMP levels in an attempt to benefit a greater portion of patients undergoing thiopurine treatment. Although the relationship between 6-TGN levels and efficacy was first described in patients with IBD in 1996,109 this correlation was confirmed in the landmark study in 2000 by Dubinsky et al.,112113 which demonstrated a positive correlation of therapeutic response with 6-TGN levels in 92 pediatric patients with IBD. The frequency of therapeutic response increased at 6-TGN levels >235 pmol/8×108 red blood cells (RBC) while hepatotoxicity correlated with elevated 6-MMP levels (>5700 pmol/8×108 RBC).113 Since then, several prospective studies have reported a correlation between 6-TGN levels and clinical response.114115116117 A meta-analysis of 12 studies with 941 patients concluded that patients with 6-TGN levels above 230-260 pmol/8×108 RBC were more likely to be in remission than those below the threshold value.8 A more recent pooled analysis including 17 studies of 2,049 patients showed that the pooled OR for clinical remission among patients with 6-TGN levels over a cut-off value between 230 and 260 pmol/8×108 RBC was 3.15.118 Although the dose adjustment of thiopurines through monitoring of 6-TGN and 6-MMP levels is expected to improve efficacy and reduce side effects in patients treated with thiopurines, further studies are needed in order to confirm these findings.
CONCLUSIONS
For decades, thiopurine agents have been a mainstay in treating IBD and will play an important role in the future. However, complex metabolism and various side effects limit their successful application in clinical practice. Thiopurine-induced leukopenia is especially very common and prescreening for TPMT has a limited value in Korean patients with IBD. Gradual dose increment with careful monitoring of CBC is essential to reduce this complication. Maintaining with low dose of thiopurines can be a great alternative. In addition, use of novel biomarkers such as NUDT15, may help identify patients who are at a high risk for thiopurine-induced leukopenia. At present, optimal duration of thiopurine treatment in addition to long-term outcomes of combination therapy with anti-TNF agents is uncertain. Management decisions should be individualized based on the risk of relapse and adverse events. Although thiopurine treatment during pregnancy and lactation is considered safe and well tolerated, it is important to balance the risks and benefits.
Notes
Financial support: This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120176).
Conflict of interest: None.