Safety and effectiveness of adalimumab in the treatment of ulcerative colitis: results from a large-scale, prospective, multicenter, observational study
Article information
Abstract
Background/Aims
Adalimumab has been shown to induce and maintain clinical remission in patients with moderate to severe ulcerative colitis (UC). However, no large-scale population-based studies have been performed in Japan. This study was conducted to evaluate the safety and effectiveness of adalimumab in clinical practice in Japanese patients with UC.
Methods
In this 52-week, prospective, multicenter, single-cohort, noninterventional, observational, postmarketing surveillance study, patients with moderate to severe UC received an initial subcutaneous injection of adalimumab 160 mg, followed by 80 mg at 2 weeks, and then 40 mg every other week. Safety assessments were the incidence of adverse drug reactions (ADRs) and serious ADRs. Effectiveness assessments were clinical remission, corticosteroid-free remission, mucosal healing, and change in C-reactive protein (CRP) levels from baseline.
Results
Of 1,593 registered patients, 1,523 (male, 57.6%; mean age, 41.8 years) and 1,241 patients were included in the safety and effectiveness populations, respectively. ADRs were reported in 18.1% and serious ADRs in 4.9% of patients. Clinical remission was achieved in 49.7% of patients at week 4, increasing to 74.4% at week 52. Corticosteroid-free remission rates increased over time, from 10.4% at week 4 to 53.1% at week 52. More than 60% of patients demonstrated mucosal healing at weeks 24 and 52. Mean CRP levels (mg/dL) decreased from 1.2 at baseline to 0.6 at week 4 and 0.3 at week 52.
Conclusions
This large real-world study confirmed the safety and effectiveness of adalimumab in patients with UC in Japan. No new safety concerns were identified.
INTRODUCTION
Ulcerative colitis (UC) is a nonspecific, idiopathic, inflammatory bowel disease characterized by persistent diffuse inflammation in the colonic mucosa that frequently results in ulceration in the colon and rectum [1,2]. Typically, ulceration starts in the rectum and extends upward through part of, or the entire, colon [1,2]. Characteristic symptoms of UC include bloody diarrhea, urgency, tenesmus, abdominal pain, and, occasionally, fever. The clinical course of UC is unpredictable, marked by alternating cycles of relapse and remission [2,3].
The highest annual occurrence of UC has been reported in Europe (24.3 per 100,000 person-years) and North America (19.2 per 100,000 person-years) [4]; the annual occurrence in Asian and Middle-East countries (6.3 per 100,000 personyears) is lower than in Western countries [4]. However, a recent report highlighted an increasing incidence of UC across Asia in parallel with rapid urbanization, with the highest incidence generally observed in Korea, Japan, China, Hong Kong, and India [5]. Notably, from 1991 to 2005 the prevalence of UC in Japan increased from 18.1 to 63.6 per 100,000 persons [6,7], rising to approximately 100 per 100,000 persons in 2010 [8]. In Japan, the peak age of UC onset is 20 to 29 years for both sexes, with the disease equally prevalent in men and women [9].
The main treatment goal for UC is to maintain steroid-free clinical remission in the long term [10]. Moreover, mucosal healing as an additional therapeutic goal has been shown to improve long-term disease prognosis [11]. Depending on the severity of the inflammation and the area of the gut affected, therapeutic options include 5-aminosalicylic acid, corticosteroids, and immunomodulators [1]. However, many patients experience a lack of efficacy or exhibit poor tolerability to these agents [12]. Elevated levels of tumor necrosis factor-α (TNF-α), a crucial cytokine that drives inflammatory processes, have been observed in the serum, stools, and mucosa of patients with UC [13,14]. Consequently, patients with an inadequate response or intolerance to conventional therapies have been successfully switched to treatment with anti-TNF-α antibodies to induce and maintain clinical remission [3,8,12,15-19].
Adalimumab, a fully human monoclonal antibody that targets TNF-α, was proven to be effective and safe in clinical trials of up to 4 years in patients in Western countries with moderate to severe UC who were unresponsive to traditional treatments or discontinued therapy because of safety concerns [8,12,16,17,19-22]. In addition, results from a 52-week, phase 2/3, randomized, double-blind study demonstrated the safety and efficacy of adalimumab (an initial subcutaneous injection of 160 mg, followed by 80 mg at 2 weeks, then 40 mg every other week from week 4 through 52 [160/80-mg schedule]) in anti-TNF-naive Japanese patients with moderate to severe UC who were refractory to corticosteroids, immunomodulators, or both [18]. Furthermore, an open-label extension of this study in 190 patients reported that the efficacy of adalimumab was maintained for up to 4 years, with no new safety signals identified [3].
To date, no large-scale population-based studies have been performed in Japanese patients with UC treated with adalimumab in clinical practice. Therefore, we conducted an observational study to evaluate the real-world safety and effectiveness of adalimumab in clinical practice in Japanese patients with moderate to severe UC through an analysis of postmarketing surveillance data. We also performed a post hoc analysis to examine potential factors that may affect the safety and effectiveness of adalimumab.
METHODS
1. Study Design
This 52-week, multicenter, single-cohort, noninterventional, prospective, observational study evaluated the safety and effectiveness of adalimumab in Japanese patients with moderate to severe UC. The study was conducted at 392 medical institutions in Japan between July 2013 and March 2018.
The protocol was approved by the Japanese Pharmaceuticals and Medical Devices Agency before study initiation. All registered medical institutions adhered to Good Post-marketing Study Practice (GPSP) in Japan, and the trial is registered at ClinicalTrials.gov (NCT01947816). Because the study complied with the GPSP guidance, institutional review board approval and written informed patient consent were not required.
2. Patients
Japanese patients with moderate to severe UC who had failed to respond to or who could not tolerate treatment with conventional agents and had been prescribed adalimumab were enrolled in the study. Adalimumab was administered in the 160/80-mg schedule.
3. Data Collection
Throughout the study period, investigational data were collected using a paper-based case report form (CRF). For patients who discontinued adalimumab treatment prematurely and withdrew from the study, the last available data were captured in the CRF before study discontinuation. The CRF also captured reasons for discontinuation, such as onset of an adverse event (AE), inadequate efficacy, patient request, or patient lost to follow-up.
4. Safety Assessments
The safety analysis population included all enrolled patients except those for whom CRFs were not collected, those who reported a protocol deviation, those with missing safety data, those who were not administered adalimumab or had a history of adalimumab administration or initiated adalimumab prior to contract, and those not confirmed by the physician. Safety outcomes, including adverse drug reactions (ADRs) and serious ADRs, were evaluated up to week 52 or at study discontinuation. AEs were coded with preferred terms using the bilingual (English-Japanese) edition of the Medical Dictionary for Regulatory Activities version 20.1 and classified by system organ class (SOC). ADRs were defined as AEs for which a causal relationship with the study drug could not be excluded by the physicians.
Following a univariate analysis that identified potential factors associated with the occurrence of ADRs and serious ADRs, a multivariate analysis tested these factors. The following subgroups were used in the analysis: age (< 15, 15 to < 65, and ≥ 65 years); sex; disease duration ( < 2, 2 to < 10, and ≥ 10 years); comorbidity; history of allergy or smoking; prior use of biologics, tacrolimus, or cyclosporine; concomitant use of corticosteroids or immunomodulators; and partial Mayo score at baseline (0 to < 3, 3 to < 6, and 6 to ≤ 9).
5. Effectiveness Assessments
The effectiveness population included all patients from the safety population for whom effectiveness data were also available. Effectiveness endpoints were clinical remission defined by the partial Mayo score ( ≤ 2 points, with no individual subscore > 1 point), corticosteroid-free remission, mucosal healing as assessed by endoscopy (Mayo endoscopic subscore [12] ≤ 1), and change in C-reactive protein (CRP) levels from baseline. Clinical remission and corticosteroid-free remission were evaluated at baseline and weeks 4, 8, 16, 24, and 52 and assessed as observed cases. In addition, data were recorded from patients who were not in clinical remission at week 8 but who responded to therapy and achieved remission at week 52, as well as patients who achieved remission at week 8 and at the final assessment at week 52. Endoscopic evaluations were performed at baseline and weeks 24 and 52, and changes in CRP levels were assessed at weeks 4, 24, and 52.
Following a logistic regression model that identified potential factors affecting the effectiveness of adalimumab, a multivariate analysis examined the effect of these factors on clinical remission at week 52. The following subgroups were used in the analysis: age ( < 15, 15 to < 65, and ≥ 65 years); body weight ( < 30, 30 to < 40, 40 to < 50, 50 to < 60, and ≥ 60 kg); disease duration ( < 2, 2 to < 10, and ≥ 10 years); prior use of biologics, tacrolimus, or cyclosporine; surgical history; concomitant use of corticosteroids or immunomodulators; Montreal classification; partial Mayo score at baseline (0 to < 3, 3 to < 6, and 6 to ≤ 9); and patient hospitalization status (inpatient or outpatient).
6. Statistical Analysis
Rates of clinical remission were reported as both nonresponder imputation and as-observed analyses. Potential predictive factors affecting the safety (ADRs and serious ADRs) and effectiveness (clinical remission) of adalimumab were identified using a logistic regression model. A stepwise selection procedure was then used to determine the factors to be included in the final model. Depending on the variable, the Fisher exact test or the Mann-Whitney U test (age, body weight, disease duration, and partial Mayo score) was used for statistical comparisons. Multivariate analyses of factors associated with safety (ADRs and serious ADRs) and effectiveness (clinical remission at week 52) were performed in a post hoc analysis. All tests were performed with a 2-sided significance level of 5% and conducted using SAS System Release 9.1 (SAS Institute Inc., Cary, NC, USA), with P<0.05 defining statistical significance.
RESULTS
1. Patient Disposition and Baseline Characteristics
Overall, 1,621 patients were enrolled in the study, with CRFs available for 1,593 patients. Following the exclusion of 70 patients, 1,523 patients were included in the safety analysis population (Fig. 1). Of these patients, effectiveness data were not available for 282 patients; therefore, 1,241 patients constituted the effectiveness analysis population (Fig. 1).

Patient disposition. aReasons for exclusion were overlapped when data were aggregated. CRF, case report form.
Patients in the safety analysis population had a mean ± standard deviation exposure to adalimumab of 266.9 ± 135.5 days (median, 365 days). The dose was escalated in 12 of 1,241 (1.0%) patients in the effective analysis population and 18 of 1,523 (1.2%) patients in the safety analysis population. Overall, 940 (61.7%) patients continued adalimumab therapy until week 52; 583 patients discontinued treatment before study end, most commonly because of an inadequate response (59.0%, 344/583) and AEs (16.0%, 93/583).
Patient demographics and baseline characteristics of the safety analysis population are shown in Table 1. Overall, 57.6% (878/1,523) of patients were men, and the mean age was 41.8 years. Patients had a mean duration of UC of 7.9 years and a mean baseline partial Mayo score of 5. Approximately twothirds (67.6%, 1,029/1,523) of patients were nonsmokers and 42.7% (650/1,523) of patients had comorbidities. The use of concomitant corticosteroids was reported in 46.6% (709/1,523) and immunomodulators (azathioprine and 6-mercaptopurine) in 43.6% (664/1,523) of patients. Prior use of infliximab was reported in 25.6% (390/1,523) of patients. According to Montreal classification, 66.8% of patients had extensive colitis, and 2.4% of patients had disease limited to the rectum.
2. Safety
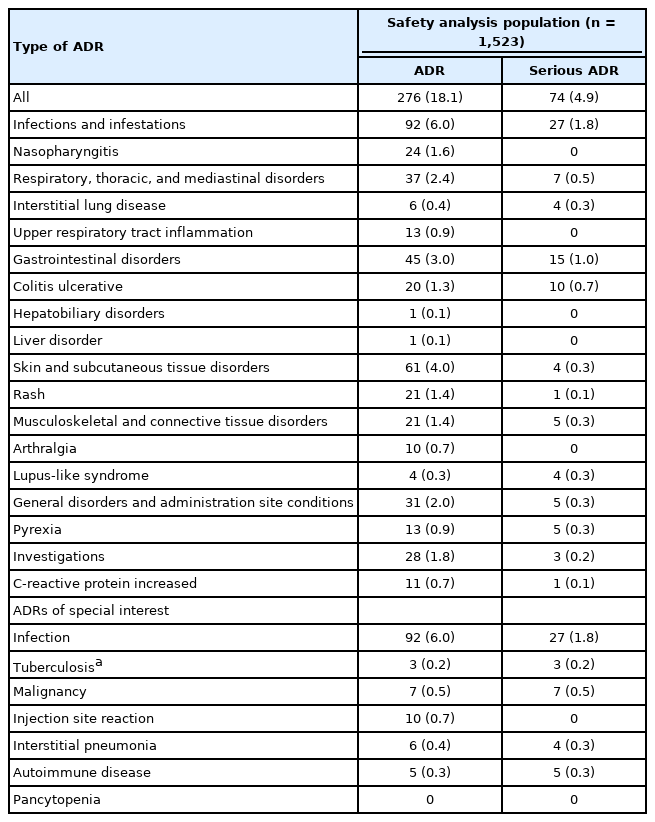
In the safety analysis set, 408 ADRs were reported in 18.1% (276/1,523) of patients. An overview of ADRs, serious ADRs, and ADRs of special interest is presented in Table 2. The most common ADRs by SOC ( ≥ 25 patients) were infections and infestations; skin and subcutaneous tissue disorders; gastrointestinal disorders; respiratory, thoracic, and mediastinal disorders; general disorders and administration site conditions; and investigations (Table 2). By preferred term, the most common ADRs ( ≥ 10 patients) were nasopharyngitis, rash, colitis ulcerative, pyrexia, upper respiratory tract inflammation, arthralgia, and CRP increased (Table 2). Overall, 95 serious ADRs were reported in 4.9% (74/1,523) of patients. The most common serious ADRs by SOC ( ≥ 10 patients) were infections and infestations and gastrointestinal disorders, and by preferred term ( ≥ 3 patients) were colitis ulcerative, pyrexia, interstitial lung disease, and lupus-like syndrome (Table 2). No cases of de novo or reactivation of hepatitis B were reported.
During treatment with adalimumab, tuberculosis was reported in 3 patients (0.2%, 3/1,523). All 3 patients had no history of tuberculosis or use of biologics and were receiving corticosteroids and 5-aminosalicylic acid at the time of adalimumab initiation. Two patients, 40 and 51 years of age, had pulmonary tuberculosis on days 55 and 247 of treatment with adalimumab, respectively. The causality of pulmonary tuberculosis in the 40-year-old patient was judged by the investigator as related to the study drug, whereas in the 51-year-old patient the causality with adalimumab was not excluded. A third patient, 55 years of age, was diagnosed with disseminated tuberculosis on day 199 of adalimumab treatment; causality of tuberculosis was judged to be related to the study drug in the investigator’s opinion. All 3 cases were considered serious. At the last follow-up, the case of disseminated tuberculosis (35 days after reporting) and the case of pulmonary tuberculosis in the 40-year-old patient (78 days after reporting) were reported as resolved or improved, whereas the status of the 51-year-old patient with pulmonary tuberculosis (43 days after reporting) was unknown. All 3 patients underwent tuberculosis screening before initiation of adalimumab treatment, with chest radiographs reported as normal in all 3 patients and a negative interferon-gamma release assay result reported in 2 of the 3 patients (the result for 1 of the patients with pulmonary tuberculosis was indeterminate).
Malignancy was reported in 10 patients (0.7%); 3 of these cases (colon cancer, breast cancer, and hepatocellular carcinoma) were not considered related to adalimumab treatment. Overall, the ADRs were colon cancer (2 patients), breast cancer (1 patient), lung cancer (2 patients), keratoacanthoma (1 patient), and rectal cancer (1 patient). At follow-up, 4 of the 10 cases (2 colon, 1 lung, and 1 hepatocellular carcinoma) of malignancy were reported as resolved. The outcome at follow-up was not resolved for a case of rectal cancer and unknown for a case of colon cancer. The patient with breast cancer died 61 days after the first injection of adalimumab. The condition of a patient with lung cancer deteriorated 260 days after the first injection of adalimumab and the patient died 15 days later. Follow-up information was either not provided or unknown for the patient with keratoacanthoma. Both patients with colon cancer (colon cancer stage I and adenocarcinoma of colon) received concomitant immunomodulator therapy during the study. Autoimmune disease was reported as a serious ADR in 5 female patients (age range, 44–64 years): systemic lupus erythematosus in 1 patient and lupus-like syndrome in the remaining 4 patients. Autoimmune disease reportedly resolved in 3 patients, improved in 1 patient, and remained unresolved in 1 patient.
Multivariate Analysis
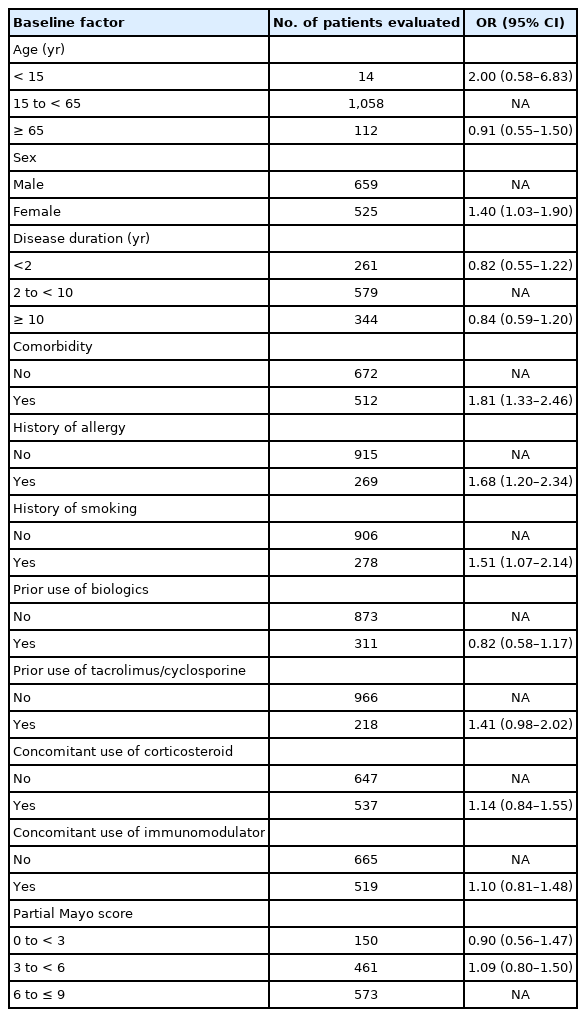
An assessment of the effect of patient baseline factors on the onset of ADRs and serious ADRs is shown in Table 3. The multivariate and subgroup analysis in 1,184 patients identified the following factors as affecting the safety of adalimumab therapy: female sex (odds ratio [OR], 1.40; 95% confidence interval [CI], 1.03–1.90), presence of comorbid conditions (OR, 1.81; 95% CI, 1.33–2.46), history of allergy (OR, 1.68; 95% CI, 1.20–2.34), and history of smoking (OR, 1.51; 95% CI, 1.07–2.14).
3. Effectiveness
At weeks 4 and 52, 49.7% (531/1,068) and 74.4% (539/724) of patients achieved clinical remission, respectively (Fig. 2A). The majority of patients who achieved a clinical response (according to the partial Mayo score) at week 8 also achieved clinical remission at week 52 (80.7% [330/409]; 95% CI, 76.5%–84.4%). Similarly, most patients in clinical remission at week 8 maintained their remission status at week 52 (86.1% [272/316]; 95% CI, 81.8%–89.7%). Of 539 patients who achieved clinical remission at week 52, 78.1% were in remission as early as 8 weeks after initiating treatment with adalimumab. However, adalimumab induced clinical remission for the first time in < 10% of patients between weeks 24 and 52.

(A) Rates of clinical remission per partial Mayo score in the effectiveness analysis population (observed cases). Clinical remission was defined by a partial Mayo score ≤2 points with no individual subscore >1 point. (B) Rates of corticosteroid-free remission in patients initiating adalimumab with concomitant corticosteroid therapy (observed cases). (C) Rates of mucosal healing in the effectiveness analysis population (observed cases); mucosal healing was defined as a Mayo endoscopic subscore ≤1.
In 504 patients who received corticosteroids at the start of adalimumab treatment, corticosteroid-free remission rates increased from 10.4% (51/489) at week 4 to 32.9% (124/377) at week 24 and 53.1% (171/322) at week 52 (Fig. 2B).
Mucosal healing was reported in 63.9% of patients at week 24 (Mayo endoscopic subscore 0 and 1, 29.5% and 34.4%, respectively) and 67.6% of patients at week 52 (34.3% and 33.3%) (Fig. 2C).
In the 1,058 patients who underwent CRP assessments at baseline, the mean ± standard deviation CRP level was 1.2 ± 2.4 mg/dL. Following initiation of adalimumab treatment, CRP levels decreased at weeks 4 (0.6 ± 1.7 mg/dL; n = 882), 24 (0.4 ± 1.0 mg/dL; n = 533), and 52 (0.3 ± 0.9 mg/dL; n = 597).
Multivariate Analysis
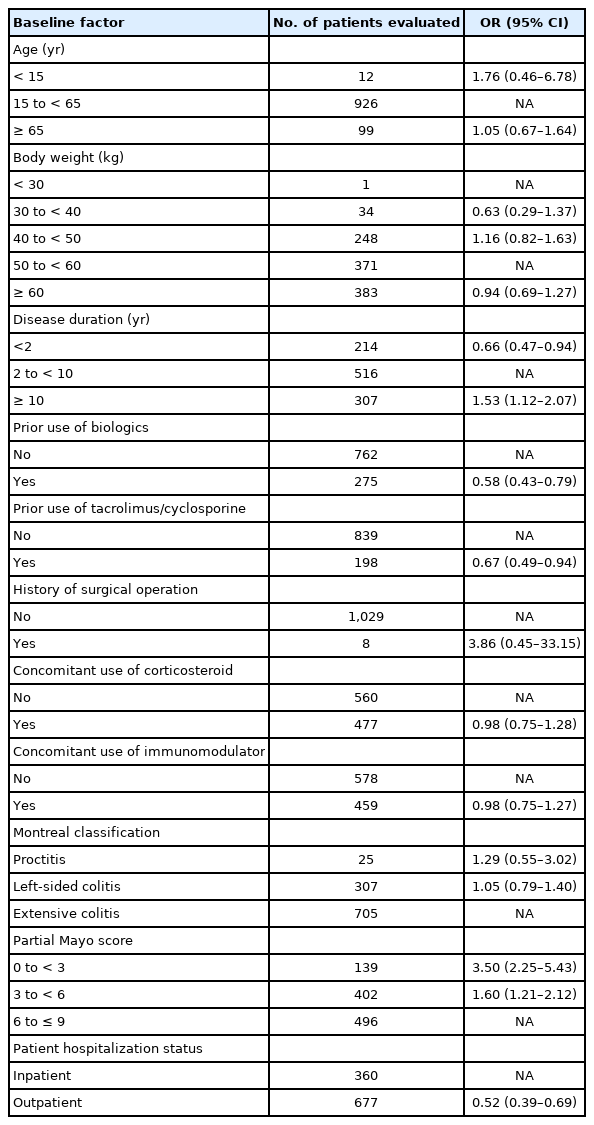
An assessment of the impact of patient baseline factors on the effectiveness (clinical remission) of adalimumab is shown in Table 4. Results from the multivariate and subgroup analysis in 1,037 patients identified the following factors as affecting the attainment of clinical remission at the last evaluation: disease duration < 2 years (OR, 0.66; 95% CI, 0.47–0.94) and duration ≥ 10 years (OR, 1.53; 95% CI, 1.12–2.07), previous use of tacrolimus/cyclosporine (OR, 0.67; 95% CI, 0.49–0.94) or biologics (OR, 0.58; 95% CI, 0.43–0.79), partial Mayo score 0 to < 3 (OR, 3.50; 95% CI, 2.25–5.43) and 3 to < 6 (OR, 1.60; 95% CI, 1.21–2.12), and outpatient status (OR, 0.52; 95% CI, 0.39–0.69).
DISCUSSION
This study is the first large-scale, real-world study to investigate the safety and effectiveness of adalimumab in Japanese patients with UC. Overall, the safety profile of adalimumab was consistent with that previously reported in clinical studies in both Japanese and Western populations with UC [3,8,12,16-18]. Moreover, this study did not identify any new safety signals. The most frequent serious ADR by SOC was infections and infestations.
An increased risk of tuberculosis in patients treated with TNF-α antagonists is well documented [23]. Therefore, evidence-based guidelines recommend screening procedures, such as interferon-gamma release assay, tuberculin skin test, and chest radiograph, before starting treatment with anti-TNF agents [1]. Consequently, tuberculosis was an ADR of particular interest in this study. During treatment with adalimumab, tuberculosis was reported in 3 patients, a finding in accordance with previous studies that examined the use of adalimumab in patients with UC or Crohn’s disease [3,18,24]. These patients were using concomitant corticosteroids at baseline, and it is known that high-dose prednisolone is associated with unreliable results on the QuantiFERON® (Qiagen; Hilden, North RhineWestphalia, Germany) test, which was used for tuberculosis screening in this study [25]. Therefore, in patients who receive high-dose corticosteroids, the results of the QuantiFERON® test need to be interpreted with caution, and a chest computed tomography scan may be considered as an alternative for tuberculosis screening.
Although the adalimumab prescribing information states that tuberculosis screening is a requirement before the initiation of adalimumab therapy [26], only 77% of patients in the safety analysis set underwent tuberculosis screening in this study. This could be because patients who had previously received treatment with an anti-TNF-α agent had already undergone tuberculosis testing before receiving adalimumab. Before initiation of therapy with adalimumab, patients should be evaluated for active or latent tuberculosis infection [27,28]. Patients with previous or recent biologic use should not be exempted from tuberculosis screening tests.
Malignancy, associated with adalimumab, was reported in 7 patients in this study, but no clear trend in cancer type or association with disease duration, concomitant medications, or smoking habits could be established. Of these patients, 3 who developed cancer of the colon and/or rectum had a long disease duration ( ≥ 9 years), which was previously reported as a risk factor for colorectal cancer [29], and 1 patient had breast cancer at baseline. Three patients, 2 of them with colorectal cancer, were receiving concomitant immunomodulators at baseline, and several analyses have demonstrated an increased risk of lymphoproliferative disorders with the use of thiopurines [30,31]. Thus, 6 of 7 patients who reported a malignancy during adalimumab treatment had potential risk factors such as a previous malignancy, long disease duration, or concomitant use of azathioprine. Therefore, it might be important to closely monitor patients with potential risk factors that have been associated with the onset of malignancy in patients with UC [32].
An assessment of patient baseline factors revealed that female sex, presence of comorbidities, and history of allergy or smoking all affected the safety profile of adalimumab treatment in terms of increasing the likelihood of ADRs and serious ADRs. Interestingly, smoking, at least at low levels [33], is reported to be protective against UC, with results from a small study in 17 ex-smokers demonstrating that low-dose smoking resumption in patients with refractory UC may ameliorate signs and symptoms of inflammation; however, its causality has not been clear. In contrast, mice studies have linked smoking with UC-associated colorectal cancers [34,35], and smoking may increase the risk of thromboembolic complications in patients with UC [36]. Consequently, the causal association between smoking and UC remains undetermined, and further research is warranted [1].
The present trial also demonstrated the long-term effectiveness of adalimumab therapy in Japanese patients with moderate to severe UC. According to the partial Mayo scores, patients achieved clinical remission as early as week 4, with more than 70% of patients reporting no signs of relapse at week 52. Notably, the majority of patients who achieved a clinical response or were in clinical remission (per the partial Mayo score) at week 8 reported sustained effectiveness until week 52. These findings are in accordance with those from the 52-week, phase 2/3, randomized, double-blind study in Japan in which the majority of patients who achieved a clinical response (per the partial Mayo score) at week 8 attained remission at week 52 [18]. These findings were also replicated in the open-label extension of that trial, which reported that clinical response and remission per full Mayo score were achieved early (week 8) and sustained throughout the 4-year study [3]. In addition, an assessment of patient baseline factors revealed a number of factors affecting the effectiveness (attainment of clinical remission) of adalimumab, including disease duration < 2 years or ≥ 10 years and a partial Mayo score of 0 to < 3 or 3 to < 6.
Corticosteroids were among the first agents to be used in the management of UC [1]; however, their continued use is not recommended because they do not have a significant long-term disease-modifying effect and are associated with considerable AEs, including corticosteroid-induced ecchymosis, infections, acne, moon face, hirsutism, petechial bleeding, and striae [37]. Moreover, their long-term use can cause hypertension, new-onset diabetes mellitus, infection, osteonecrosis, corticosteroid-associated osteoporosis, myopathy, psychosis, cataracts, and glaucoma [37]. Consequently, corticosteroid-free remission has become an important therapeutic goal when selecting a treatment for UC and was one of the endpoints assessed in this study. Over the course of this study, adalimumab therapy was associated with a meaningful reduction in the concomitant use of corticosteroids in patients taking corticosteroids at baseline, with more than 50% of patients with corticosteroid-free remission at week 52; this observation was comparable to that reported in the phase 2/3, randomized, double-blind study in Japan, where approximately 47% of patients were reported as corticosteroid-free at week 52 [18].
A 16-week study in patients with UC demonstrated that anti-TNF-α-naive patients showed a higher rate of mucosal healing and corticosteroid-free remission with a combination of infliximab and azathioprine compared with either drug administered as monotherapy [38]. In this study, multivariate analysis did not suggest that concomitant use of immunomodulators, including azathioprine and 6-mercaptopurine, was a predictor of improved efficacy. However, future prospective studies to investigate a combination of azathioprine and adalimumab in patients with UC are warranted.
Traditionally, the treatment of UC has focused on the management of clinical symptoms. However, the treatment paradigm for UC has recently shifted toward objective measures, with results from a recent meta-analysis of 13 studies in 2,073 patients with active UC confirming that mucosal healing is an appropriate goal of UC therapy because of its association with long-term clinical remission, corticosteroid-free clinical remission, and avoidance of colectomy [11]. In the present study, a substantial proportion of patients ( > 60%) achieved mucosal healing at week 24, which was sustained in patients who continued adalimumab therapy until week 52.
The assessment of a biomarker, such as CRP, provides a meaningful measure of inflammation in patients with UC [39]. Following adalimumab treatment, mean serum CRP levels consistently decreased from baseline at all evaluated time points, demonstrating biologic anti-inflammatory activity.
This study has several strengths. Notably, the long-term safety and effectiveness of adalimumab was evaluated in a large population of patients with UC in routine clinical practice. Therefore, this study provides further valuable evidence on the use of adalimumab in patients with UC in a real-world setting. However, the study also has several limitations. First, the noninterventional and observational nature of the study meant that patients were not selected according to strict inclusion criteria, specifically regarding the use of previous and concomitant medication other than biological agents. Second, due to the postmarketing nature of this study, some patients discontinued during the observation period. Finally, an assessment of ADRs by patient baseline characteristics may not have been entirely appropriate because of an imbalance in baseline characteristics between the patient subgroups. Nevertheless, we believe the results of this study reflect the use of adalimumab for the treatment of UC in daily clinical practice in Japan.
In conclusion, this large real-world study demonstrated the safety and effectiveness of adalimumab for the treatment of moderate to severe UC in Japanese patients. Overall, the safety profile was similar to that reported in previous clinical trials, with no new safety concerns identified. In addition, adalimumab treatment led to long-term response, remission, and mucosal healing.
Notes
Funding Source
This study was funded by AbbVie GK and Eisai Co., Ltd.
Conflict of Interest
Ogata H reports receiving financial support for research from Eisai Co. and lecture fees, consultancy fees, and other support from AbbVie GK. Hagiwara T holds stocks/stocks options of AbbVie Inc. and is an employee of AbbVie GK. Kawaberi T and Kobayashi M are employees of AbbVie GK. Hibi T reports receiving financial support for research and lecture fees from AbbVie GK and Eisai Co., Ltd, consultancy fees from AbbVie GK and EA Pharma, and other support from AbbVie GK.
Hibi T is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author Contribution
Conceptualization, supervision: Ogata H, Hagiwara T, Hibi T. Data curation, formal analysis, funding acquisition, investigation, resources, visualization: Hagiwara T. Project administration: Hagiwara T, Kobayashi M. Methodology, validation, writing - original draft: Hagiwara T, Kawaberi T, Kobayashi M. Writing - review and editing: all authors. Approval of final manuscript: all authors.
Others
This study was funded by AbbVie GK and Eisai Co., Ltd. The sponsors participated in the study design; data collection; analysis and interpretation of data; and writing, reviewing, and approval of the publication. All named authors meet the International Committee of Medical Journal Editors criteria for authorship, take responsibility for the integrity of the work, and give their approval for this version to be published. The standard operation procedures manual for data management and transformation was developed by A2 Healthcare Corporation and approved by the Post-Marketing Surveillance group at AbbVie, funded by the sponsors. Medical writing support was provided by Avinash Thakur and Nicola West of Cactus Life Sciences (part of Cactus Communications) and funded by AbbVie GK.