 |
 |
- Search
| Intest Res > Volume 19(3); 2021 > Article |
|
Abstract
Background/Aims
Methods
Results
ADDITIONAL INFORMATION
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
Gao X and Qian J are editorial board members of the journal but were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author Contribution
Conceptualization: all authors. Data curation: Liu J. Formal analysis: Liu J, Cao Q. Investigation: Liu J, Hu P, Cao Q. Methodology: Liu J, Zhu L, Hu P, Cao Q. Project administration: all authors. Resources: Gao X, Chen Y, Mei Q, Zhu L, Qian J, Hu P, Cao Q. Software: Liu J. Supervision: Gao X, Mei Q, Zhu L, Qian J, Hu P, Cao Q. Validation: Liu J. Visualization: Liu J. Writing - original draft: Liu J. Writing - review & editing: Liu J, Gao X, Chen Y, Hu P, Cao Q. Approval of final manuscript: all authors.
Others
We give our thanks to the following doctors for their help with data collection: Ying Han, Feng Tian, Hu Zhang, Xiaolan Zhang, Yinglei Miao, Xiaofeng Yu, Huaxiu Shi, Chengdang Wang, Yulan Liu, Xiaoyan Wang, Xuefeng Li, Chunxiao Chen, Xiaoqi Zhang, Lanxiang Zhu, Yufang Wang, Yan Zhang, Changqing Zheng, Meifang Huang, Hongjie Zhang, Wen Tang, Baisui Feng, Xiaowei Liu, Huixiang Yang, Xiaocang Cao, Qiaomin Wang, Xiaoping Lv, Feihu Bai, Zhihua Ran, Qifang Zhang, Junxia Li, Xinxin Zhou, Xinyan Yang, Qingfan Yang, Junrong Chen, Juan Luo, Ning Chen, Xiaolei Zhao, Wei Han, Di Guo, Yonghua Shen, Hui Li, Xia Zhao, Jiaming Zhang, Chuan Chen, Min Zhu, Yahui Guo, Ping Jiang, Yan Zhuang, Siqi Wang, Li Tian, Yanjun Chen, Jun Shen, Hong Yang, Hailian Zhang, Jiaqin Xu, Feng Zhou, Lianjie Lin, Jingjing Ma, Guanghui Lian, Dandan Zhou, Yunquan Zheng, Jintong Chen, Xiwen Liu.
Fig. 1.
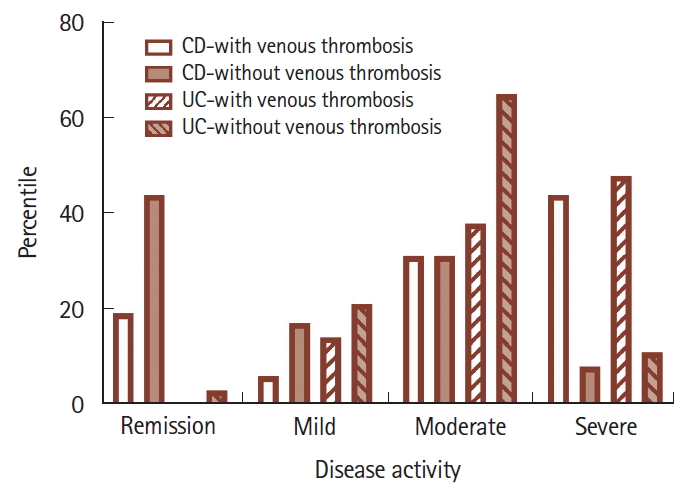
Table 1.
| Variable | With thrombosis (n = 46) | Without thrombosis (n = 8,413) | P-valuea |
|---|---|---|---|
| Male sex | 26 (56.5) | 5,282 (62.8) | 0.445 |
| Age (yr) | 46.3 ± 15.7 | 39.9 ± 15.0 | 0.004 |
| < 40 | 17 (37.0) | 4,556 (54.2) | |
| 40-59 | 18 (39.1) | 2,873 (34.1) | |
| ≥ 60 | 11 (23.9) | 984 (11.7) | |
| Type of IBD (CD) | 20 (43.5) | 4,102 (48.8) | 0.555 |
| Disease duration (mo) | 39.1 (5.3-58.5) | 21.6 (4.0-51.7) | 0.123 |
| Symptom duration (mo) | 53.4 (20.5-111.8) | 48.7 (22.1-81.2) | 0.465 |
Table 2.
| Type of thrombosis |
IBD |
CD |
UC |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | Incidencea | HR (95% CI)b | No. (%) | Incidencea | HR (95% CI)b | No. (%) | Incidencea | HR (95% CI)b | |
| All | |||||||||
| All age | 46 (0.54) | 37.18 | - | 20 (0.49) | 30.75 | - | 26 (0.60) | 44.31 | - |
| < 40 yr | 17 (0.37) | 25.50 | 1 | 9 (0.31) | 19.97 | 1 | 8 (0.49) | 37.05 | 1 |
| 40-59 yr | 18 (0.62) | 43.39 | 1.658 (0.853-3.222) | 7 (0.71) | 44.09 | 1.939 (0.718-5.234) | 11 (0.58) | 42.96 | 1.179 (0.474-2.934) |
| ≥ 60 yr | 11 (1.11) | 70.62 | 2.776 (1.294-5.955)e | 4 (1.97) | 97.65 | 3.779 (1.123-12.715)d | 7 (0.88) | 60.98 | 1.762 (0.634-4.903) |
| DVT | |||||||||
| All age | 28 (0.33) | 22.63 | - | 13 (0.32) | 19.98 | - | 15 (0.35) | 25.56 | - |
| < 40 yr | 11 (0.24) | 16.50 | 1 | 6 (0.20) | 13.31 | 1 | 5 (0.31) | 23.16 | 1 |
| 40-59 yr | 8 (0.28) | 19.29 | 1.122 (0.451-2.795) | 4 (0.41) | 25.20 | 1.660 (0.466-5.908) | 4 (0.21) | 15.62 | 0.680 (0.182-2.533) |
| ≥ 60 yr | 9 (0.90) | 57.78 | 3.343 (1.380-8.102)e | 3 (1.48) | 73.24 | 4.738 (1.173-19.134)d | 6 (0.76) | 52.27 | 2.292 (0.697-7.540) |
| PE | |||||||||
| All age | 7 (0.08) | 5.66 | - | 4 (0.10) | 6.15 | - | 3 (0.07) | 5.11 | - |
| < 40 yr | 2 (0.04) | 3.00 | 1 | 1 (0.03) | 2.22 | 1 | 1 (0.06) | 4.63 | 1 |
| 40-59 yr | 3 (0.10) | 7.23 | 2.1172 (0.361-13.054) | 2 (0.20) | 12.60 | 4.457 (0.394-50.406) | 1 (0.05) | 3.91 | 0.793 (0.050-12.715) |
| ≥ 60 yr | 2 (0.20) | 12.84 | 3.978 (0.556-28.481) | 1 (0.49) | 24.41 | 5.682 (0.293-110.140) | 1 (0.13) | 8.71 | 1.708 (0.106-27.457) |
| Otherc | |||||||||
| All age | 11 (0.13) | 8.89 | - | 3 (0.07) | 4.61 | - | 8 (0.18) | 13.63 | - |
| < 40 yr | 4 (0.09) | 6.00 | 1 | 2 (0.07) | 4.44 | 1 | 2 (0.12) | 9.26 | 1 |
| 40-59 yr | 7 (0.24) | 16.88 | 2.943 (0.860-10.069) | 1 (0.10) | 6.30 | 1.474 (0.133-16.338) | 6 (0.31) | 23.43 | 2.636 (0.532-13.064) |
| ≥ 60 yr | 0 | 0 | - | 0 | 0 | - | 0 | 0 | - |
Table 3.
Table 4.
Values are presented as mean±standard deviation or number (%). Body mass index (BMI) were missing for 2 Crohn’s disease (CD) patients with venous thrombosis; history of surgery within 3 months were missing for 1 CD patients with venous thrombosis and 2 control CD patients; history of cancer were missing for 1 CD patients with venous thrombosis and 1 control CD patients; central catheter insertion were missing for 4 control CD patients; disease behavior were missing for 3 control CD patients; current use of inflammatory bowel disease (IBD) medication were missing for 1 CD patients with venous thrombosis.
Table 5.
Values are presented as mean±standard deviation or number (%). Body mass index (BMI) were missing for 5 ulcerative colitis (UC) patients with venous thrombosis and 9 control UC patients; history of surgery within 3 months were missing for 1 UC patients with venous thrombosis and 1 control UC patients; central catheter insertion were missing for 11 control UC patients; disease severity were not assessed for 3 UC patients with venous thrombosis; past use of inflammatory bowel disease (IBD) medication were missing for 1 UC patients with venous thrombosis; current use of IBD medication were missing for 2 UC patients with venous thrombosis.








