An integrative review of physical activity in adults with inflammatory bowel disease
Article information
Abstract
Adults with inflammatory bowel disease (IBD) search for self-management strategies to manage their symptoms and improve their quality of life (QOL). Physical activity (PA) is one of the self-management strategies widely adopted by adults with IBD. This integrative review aimed to synthesize the evidence on health outcomes of PA in adults with IBD as well as to identify the barriers to engaging in PA. Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), published literature was searched to identify the articles that addressed PA in adults with IBD. Twenty-eight articles met the inclusion criteria. Many of the reviewed studies used the terms of PA and exercise interchangeably. Walking was the most common PA reported in the studies. The findings from the majority of the reviewed studies supported the benefits of moderate-intensity exercise/PA among adults with IBD. The reviewed studies noted the following positive health outcomes of PA: improvement in QOL, mental health, sleep quality, gastrointestinal symptoms, fatigue and cardiorespiratory fitness. More importantly, participation in PA reduced the risk for development of IBD and the risk for future active disease. The findings from the reviewed studies highlighted the following barriers to engage in PA: fatigue, joint pain, abdominal pain, bowel urgency, active disease and depression.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic illness comprised of 2 forms of disorders: Crohn’s disease (CD) and ulcerative colitis (UC) [1]. The incidence rate of IBD is increasing worldwide, including developing countries where IBD was previously considered a rare disorder [2]. Adults with IBD present with many disturbing gastrointestinal (GI) symptoms, such as diarrhea, abdominal pain, bowel urgency, rectal bleeding, and extraintestinal manifestations [3]. Additionally, systemic symptoms, such as anxiety, depression, fatigue, sleep disturbances, and pain, are also common in adults with IBD [4]. The quality of life (QOL) of those with IBD is lowered due to the GI and systemic symptoms, as well as due to the extraintestinal manifestations [5,6]. Although, many advanced medical and surgical therapies are available to manage IBD, adults with IBD may look for other adjuvant options to manage their symptoms and improve their QOL. Physical activity (PA) is one such alternative intervention [7]. Exercise is an equivalent term used in the literature for PA, however it refers to more structured and repetitive activities [8].
Alterations in the intestinal immune system act as a trigger for IBD inflammation [9]. The anti-inflammatory benefits of exercise are well documented and are related to the control of pro-inflammatory cytokines in the intestinal system. In healthy individuals, the immune system of the intestine is in a state of equilibrium between pro- and anti-inflammatory mechanisms. However, disturbances in this equilibrium lead to the activation of the intestinal immune system and subsequent inflammation in IBD [9]. Animal model studies show that moderate exercise downregulates cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor (TNF)-α, which reduce inflammation, suggesting a beneficial influence of exercise on IBD inflammation [9-11]. Additionally, the anti-inflammatory effects of exercise are related to the release of myokines from the muscles, such as IL-6, IL-10, and IL-1ra. These myokines inhibit TNF-α production, which reduces intestinal inflammation [10-12].
While reviews have been published on the benefits of PA for adults with IBD, reviews had mixed results and many reviews included older studies. Furthermore, many studies did not include barriers to engaging in PA/exercise among adults with IBD. Therefore, the aim of this review was to synthesize the literature on PA in adults with IBD with a focus on health outcomes of PA in adults with IBD as well as to identify the barriers to engage in PA by those with IBD.
METHODS
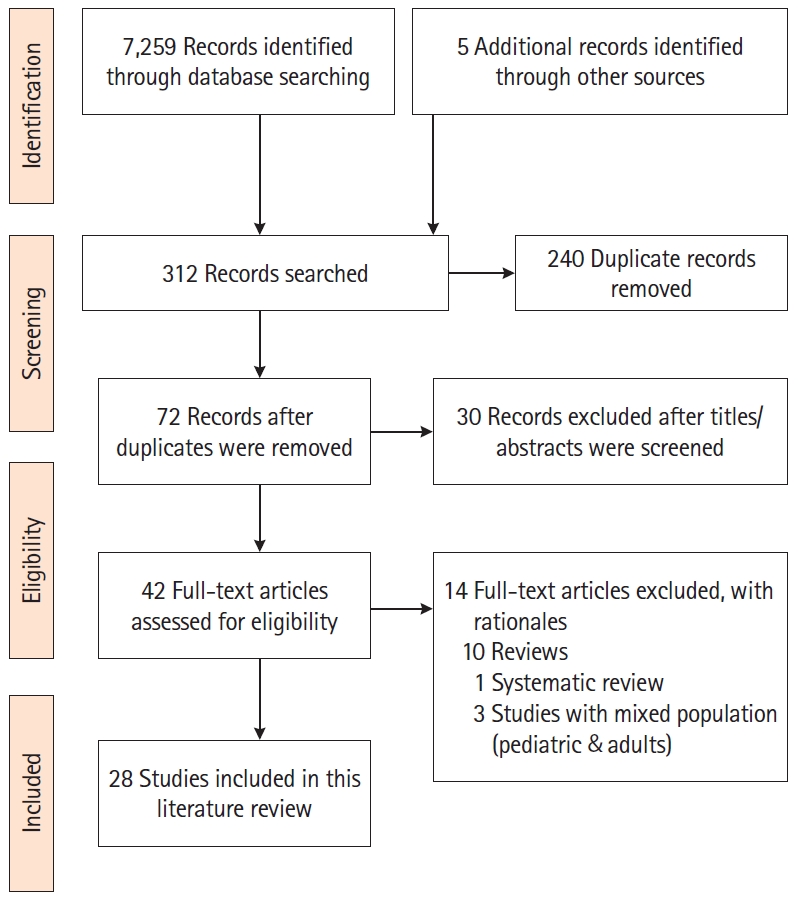
We conducted an integrative literature review from September 2019 to March 2020 to identify published articles that addressed PA/exercise in adults with IBD. The authors followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) as a framework to guide the literature search [13]. We searched the electronic databases of PubMed, CINAHL, Embase, Cochrane Controlled Trials Registry and Clinical Trials.Gov to identify the articles. The following keywords were used: “inflammatory bowel disease, Crohn’s disease, ulcerative colitis, physical activity, exercise, health outcomes, barriers, aerobic exercise, and resistance training.” The authors customized the keywords based on the requirements of each database. Additionally, the first author conducted a manual search of the selected studies to identify additional studies. The inclusion criteria of the studies were: (1) randomized and non-randomized studies, including cross-sectional studies that addressed exercise or PA; (2) studies published from 2007 to 2020; and (3) studies in English focused on adults (> 18 years) with IBD. The exclusion criteria were: (1) studies with pediatric population; (2) studies in other languages; and (3) reviews and conference abstracts. The PRISMA diagram (Fig. 1) illustrates the search process and the final selection of studies.
RESULTS
1. Characteristics of the Studies
A total of 28 eligible studies were included for review. Of these, only 4 were randomized controlled trials; the rest were cross-sectional, prospective, descriptive, and case control studies. The study settings varied including Asia, Australia, Europe, and North America with a total sample of 8,168 adults. There were 15 studies with sample sizes of more than 100 [14-28]. For other details about the studies, including the author(s), year, study type, methods, and main findings, refer to Supplementary Table 1.
2. Physical Activity Approaches
Although exercise is a subset of PA [8], many of the reviewed studies used these terms interchangeably. Twelve of the studies measured PA using self-report questionnaires. Of these studies, most used standardized tools, such as the Godin Leisure Time Questionnaire (GLTQ) [16,19,22,29] and the International Physical Activity Questionnaire (IPAQ) [27,28,30,31]. Various tools were used in the remaining studies. Details of PA measurement and characteristics were listed in Table 1.
Besides self-report, several researchers directly supervised the exercise activities of the participants. The supervised exercise activities were as follows: combined aerobic and resistance training under the supervision of a gym instructor [32]; a supervised running program [33]; progressive resistance training, which consisted of quadriceps training through leg extensions on a weight machine [26]; a cardiopulmonary exercise test [34]; evaluation of muscle strength (handgrip and quadriceps strength) using a dynamometer; the sit up test (ability to rise from a chair); and the gait speed test (walking velocity with a trainer) using a digital chronometer [35]. Additionally, high-intensity interval training (HIIT) and moderate-intensity continuous training (MICT) were also provided to adults with IBD using a leg cycle ergometer [36].
Other studies (n = 4) measured PA objectively but in an unsupervised manner. For example, one study measured a group of participants engaged in walking by having them wear pedometers [31]. In addition, 3 studies measured PA using accelerometers [37-39].
Exercise advice with the help of a PA trainer was also documented in the literature. For example, one study [40] focused on exercise advice as an intervention for adults with IBD. Each participant met with the researchers and a PA trainer for 15 minutes in week 1; advice was given to the participants on how to increase their PA by over 30% based on their personalized goals and instructions given to participants to track their PA goals and achievements in a diary [40].
3. Types of Physical Activity
The majority of the reviewed studies supported the benefits of systematic moderate-intensity exercise among adults with IBD [18,27,32-34]. However, 2 studies mentioned benefits from low-intensity physical activities [29,31]. In addition, a pilot study supported the feasibility and acceptability of high-intensity and moderate-intensity training [36]. In one study, the majority of the participants (52.9%) were engaged in high levels of PA [30]. The results of the other studies were inconclusive or did not measure the preferred intensity of PA due to their cross-sectional nature.
Walking was the most common type of PA reported [17,23,24,28,29,31]; followed by running [17,23,28,33]. Adults with IBD also participated in other physical activities, such as swimming, cycling, yoga, gardening/yardwork, home-based exercises, jogging, tennis, and gym exercise classes [23,24,28,29]. The exercise advice in one study recommended swimming, walking, and simple gym routines for less active individuals and active individuals were instructed to extend their activities, such as training for a 5–10 km run [40]. Two of the reviewed studies [26,36] provided resistance training to participants and one study offered a combination of aerobic and resistance training [32].
4. Health Outcomes of Physical Activity
Adults with IBD reported improvements in their physical and mental health outcomes, as well as better management of IBD symptoms, after initiating exercise. Majority of the reviewed studies [25-27,30,32,33,36,37,39,40] measured QOL using Inflammatory Bowel Disease Questionnaire (IBDQ). This is a validated instrument to measure disease specific QOL among adults with IBD. In addition to IBDQ, 2 studies included EuroQOL to measure QOL [36,40], and 2 other studies measured QOL using Short Form 36 (SF-36) scale [27,37]. A direct positive relationship between QOL scores and exercise were reported in many studies; One study noted significant differences in the QOL scores between the exercise and non-exercise groups (P<0.05) [31]. Another study reported a positive correlation between QOL scores and PA which was measured using an accelerometer (P=0.03) [39]. Similar results were reported after progressive resistance training in 148 women with IBD; the QOL scores significantly improved (P=0.0001) after an 8-week training period [26]. Improved mean QOL scores was noted among participants who engaged in MICT [36]. Similar results were also observed among adults with CD, where physically active adults with CD reported greater QOL (P=0.022) [30]. In a cross-sectional survey, 79.4% of participants (n = 158) reported higher QOL after initiating exercise [19]. Meanwhile, the findings of another cross-sectional analysis revealed higher QOL with higher moderate to vigorous PA (> 150 min/wk; P<0.001) and increased walking (> 60 min/wk; P<0.01) [27]. Conversely, QOL scores did not differ between the exercise and control groups in a randomized control trial designed to measure the influence of a 10 week PA program in adults with IBD [33]. However, a significant improvement (P=0.023) in the QOL social subscores was noted, which suggested that exercise can promote QOL through improvements in social well-being [33]. Details of QOL results are summarized in Table 2.
PA influenced mental health outcomes and other IBD symptoms. Two studies noted that exercise led to improvements in mood [29,36]. Significant differences in stress scores between the exercise and control groups (P<0.05) were reported in one study with reduced stress scores among the participants of the exercise group [31]. Other additional benefits of PA included improved energy and sleep quality, fewer GI symptoms, and weight control [17,19,29,36].
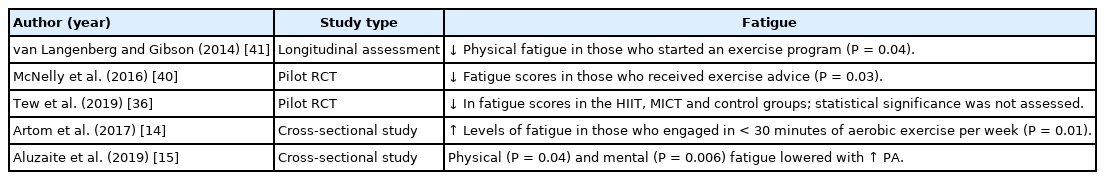
Besides the improvement of physical, mental, and IBD symptoms, PA has been found to help reduce fatigue among adults with IBD. A longitudinal assessment of 86 adults with CD identified regular exercise as a modifiable behavior to reduce physical and cognitive fatigue among participants [41]. Fatigue scores were significantly lower in those who received exercise advice compared to those who received an exercise placebo (P=0.03) [40]. Another study tested 2 forms (HIIT and MICT) of exercise among adults with CD [36]. Fatigue scores were reduced in both groups, and the control group compared to baseline, but this did not differ among the groups.36 Two cross-sectional analyses found that higher levels of PA were associated with lower fatigue levels [14,15]. Specifically, participants who engaged in exercise for less than 30 minutes in a week experienced higher levels of fatigue (P=0.01) [14]. The summary of the results on fatigue are detailed in Table 3.
A few studies have reported the influence of PA on the cardiorespiratory fitness and body composition of adults with IBD. The cardiorespiratory fitness was measured using VO2 max (maximum oxygen uptake). Improved cardiorespiratory fitness (P=0.03) was observed among participants who engaged in moderate-intensity aerobic and resistance exercise programs for 8 weeks [32]. Participants of both HIIT, and MICT reported increased VO2 max after their sessions [36]. Interestingly, no statistically significant VO2 max results were noted between adults with active and inactive IBD [34]. Results of another study found significant changes in body composition parameters (reduction in percentage of total body fat, P=0.022 and increased lean tissue mass, P=0.003) among exercise program participants compared to the control group [32]; these results also support exercise as an inexpensive intervention to manage IBD-related sarcopenia and obesity-related metabolic disorders [32].
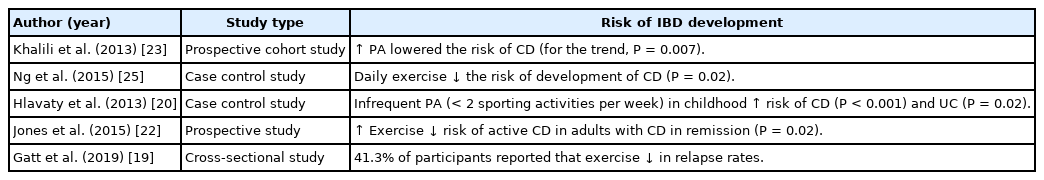
Many studies have highlighted the association between PA and the risk of developing IBD. The Nurse’s Health Study II data were evaluated to identify the association between exercise and the risk of developing IBD [23]. The PA data were collected every 2 to 4 years longitudinally in this cohort. PA was associated with a lower risk of developing CD [23]. The risk of developing CD reduced by 44% in women who did 9 hours of walking per week at an average pace. No association was noted between the risk of UC and PA [23]. The first population-based study of IBD risk factors in Asia-Pacific highlighted the protective effect of daily exercise in lowering the risk of developing CD in Asian adults with IBD [25]. Meanwhile, a Slovakian case control study reported that infrequent physical activities in childhood increased the risk of IBD [20]. In a longitudinal assessment, higher levels of exercise were significantly associated with reduced risk of active CD in adults with CD in remission (P=0.02) [22]. Additionally, 41.3% of adults with IBD (n = 158) in a cross-sectional survey (IBD diagnosed within the prior 18 months) reported that exercise helped to reduce their relapse rate [19]. Summary of the results are included in Table 4.
No definite conclusions of differences could be drawn on the health outcomes between supervised and unsupervised PA. For those studies which provided supervised PA [26,31-36,40], improvement in QOL was noted in some studies [26,31,33,36], reduced fatigue scores were noted in 2 studies [36,40], decreased total body fat, increased total lean tissue mass, and increased VO2 max in one of the studies [32]. No difference in exercise measurements were noted between participants with active IBD and inactive IBD in one supervised study [34]; and reduced lower limb performance and muscle strength were noted for adults with UC in another supervised study [35]. Similar findings were echoed in the reviewed studies which assessed PA without supervision. Improved QOL were observed in 2 of the studies [31-39]; no difference in QOL was noted in another study [37]; and lastly PA parameters were impaired among adults with CD [38].
5. Barriers to Performing Physical Activity Among Adults with IBD
Adults with IBD reported several barriers to engaging in PA. The most commonly reported barriers associated with lower PA/exercise were fatigue, joint pain, abdominal pain, bowel urgency, longer duration of disease, disease flare ups, and low vitamin D3 levels [17,18,28,38]. Depression and disease activity were negatively associated with PA in adults with CD (P<0.01), whereas depression and age were negatively associated with PA in adults with UC (P<0.01) [28]. In a study of 918 participants, 80% reported that they had to stop exercise for some period or permanently due to IBD diagnosis [17].
Analyses of the data from the research reports yielded mixed results regarding engagement in PA by adults with IBD. The results of 3 studies noted a lack of participation in exercise based on recommended guidelines [16,24,28]. Meanwhile, another study reported impaired PA parameters in adults with CD compared to healthy controls (P<0.01) [38]. Adults with CD preferred to spend their time carrying out light or sedentary activities as opposed to the recommended moderate to vigorous activity [38]. The findings of a more recent study highlighted that participants with CD and UC in remission tended to engage in higher levels of PA than those who had active disease, confirming active disease status as a barrier to engaging in PA [39]. In contrast, in another cross-sectional study, 85% of the participants with inactive IBD (n = 194) and 67% of the participants with active IBD (n = 118) were engaged in daily PA [21].
Five studies reported regular engagement in PA by more than half of the study participants [14,15,17,18,30]. The proportions of participation were: 64% (n = 182) [14], 86% (n = 113) [15], 66% (n = 918) [17], 57% (n = 17) [30], and 74% (n = 227) [18]. One cross-sectional study evaluated the physical fitness of adults with IBD in remission before and after diagnosis [19]. Adults diagnosed with IBD in the prior 18 months were included in the study. The majority of participants (69.5%, n = 158) reported that IBD diagnosis affected their PA level, whereas 30.5% did not report an effect. The diagnosis of IBD reduced participation in PA among individuals who were previously involved with sports. However, 44.3% of the participants who did not engage in sports prior to their IBD diagnosis increased their level of PA after their diagnosis [19].
A case control study discussed mild to moderate mobility limitations among adults with UC compared to age, gender, and body mass index matched healthy controls [35]. This discrepancy could be connected to evidence of the skeletal muscle changes associated with inflammation in adults with IBD [35]. Increased levels of pro-inflammatory markers increase the rate of muscle protein breakdown and reduce the rate of protein synthesis [35,42,43]. Chronic inflammation also compromises endothelial integrity, which reduces oxygen delivery to muscles [35,42,43]. In addition to muscular changes due to inflammation, IBD medications, such as corticosteroids and cyclosporine, can have a negative effect on skeletal muscles [42,44]. Based on this context of skeletal muscle dysfunction, adults with IBD should be encouraged to participate in exercise/PA to improve their muscle function, which can, in turn, result in long-term health benefits.
DISCUSSION
As evident in the literature, PA can help many chronic GI illnesses, including irritable bowel syndrome [45], diverticular diseases [46,47], and constipation [48], as well as reduce the risk of colon cancer [49]. In alignment with these studies, the reviewed research supported the benefits of PA for IBD which is a chronic illness with a disease trajectory of remission and relapse. The reviewed studies highlighted the positive effects of PA on QOL; the improvement of mental, physical, and IBD symptoms; the reduction of the risk of IBD; and the protective role of PA in maintaining remission. Overall, the reviewed studies favored low-to moderate-intensity physical activities for adults with IBD.
There are mixed opinions in published literature regarding the type and intensity of PA for adults with IBD, especially related to high-intensity exercises. In a mouse model of colitis, worsened inflammation and increased mortality were observed with forced treadmill exercise [50]. Conversely, reduced inflammatory responses in the distal colon and reduced diarrheal episodes were noted after 30 days of voluntary wheel training [50]. Intense exercise increased systemic inflammation and the level of cytokines, resulting in worsened GI symptoms [10].
However, in a qualitative analysis, participants had different views about PA [51]. Some participants were actively engaged in exercise, and they reported having more energy. Conversely, others reported a vicious circle between fatigue and exercise, where low energy prohibited them from engaging in exercise, which led to a more sedentary life [51]. Therefore, a dose-effect approach should be considered with PA/exercise in adults with IBD when considering the type of exercise, the intensity, and the duration [10]. Additionally, disease activity can be a confounding factor and patients with mild form of disease activity may engage in exercise more frequently resulting in improved IBD outcomes [11]. Although a number of reviewed studies [14,15,21,27,39] included participants with active IBD, all these studies failed to establish a positive link between PA and active disease. A number of factors can influence PA engagement during active disease including bowel urgency, toilette access and the commencement of corticosteroids and biologics [7,11,22,39]. Those with mild form of disease activity may engage in exercise more frequently resulting in improved IBD outcomes. Conversely, those with moderate or severe disease activity may have decreased frequency of PA due to pain or cramping. Further research is needed to determine PA and IBD outcomes by disease levels: mild, moderate, and severe.
None of the reviewed studies offered any specific guidelines for PA in adults with IBD. Participants of the majority of the reviewed studies engaged in walking and running as the aerobic form of PA. Although resistance training and combined aerobic exercise or resistance training were reported safe for adults with IBD, data are limited on these types of PA. Only a few studies [26,32,36] evaluated the effects of resistance training and combined aerobic exercise or resistance training in adults with IBD. However, limitations were noted in these studies including small sample sizes, and, study participants had mild disease activity or were in remission. Because participants reported that they determined the duration of exercise based on their energy levels and recommended the same strategy to others with IBD [29], we recommend individually tailored PA for adults with IBD to maximize participation and resulting PA benefits. The same notion along with the dose-effect approach [10] need to be considered while providing PA advice to adults with IBD. However, the study participants reported a lack of discussion about the health benefits of exercise from their providers [29]. The same concern was raised in another study where 46.1% of the participants (n = 158) confirmed a lack of discussion regarding exercise by their health care professionals [19].
Similar to the reviewed studies, published literature lack guidelines on PA specific for adults with IBD [22]. A recent publication [7] addressed this concern and made some recommendations based on FITT (frequency, intensity, time and type) criteria which are: taking part in moderate PA three to five times a week for at least 30 minutes (can increase the time depending on the tolerance); selecting an activity that intensifies the energy expenditure to moderate intensity is pivotal to manage inflammation; participating in both aerobic and resistance training; engaging in group PA to maximize group support and to sustain the behavior [7]. Lastly, the authors recommended future N-of -1-trials with PA due to the varying disease course of IBD with a focus on physical and psychological outcomes [7]. However, the authors solely made these recommendations based on their scoping review on PA in adults with IBD without evidence-based studies. Therefore, the applications of these recommendations are not tested. Future studies focusing the FIIT criteria and N-of -1-trials are needed to validate these recommendations in adults with IBD.
CONCLUSION
In general, the reviewed studies supported moderate-intensity PA for adults with IBD. The reviewed studies highlighted many positive health benefits of PA, such as improvements in QOL and IBD symptoms, mental health benefits, and reduced risk of development of IBD. The results of the studies also indicated many barriers to performing PA for adults with IBD. Therefore, a dose-effect approach is recommended for PA engagement among adults with IBD.
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contribution
Conceptualization, formal analysis, methodology, resources: Davis SP. Supervision: Crane PB, Bolin LP, Johnson LA. Validation: Crane PB. Writing - original draft: Davis SP. Writing - review & editing: Crane PB, Bolin LP, Johnson LA. Approval of final manuscript: all authors.
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Table 1. Details of the Reviewed Articles
ir-2020-00049-suppl.pdf