|
 |
- Search
| Intest Res > Volume 20(1); 2022 > Article |
|
Abstract
Background/Aims
Methods
Results
ADDITIONAL INFORMATION
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
Savarino EV has received lecture or consultancy fees from Takeda, Merck & Co, Bristol-Myers Squibb, AbbVie, Amgen, Novartis, Fresenius Kabi, Sandoz, Sofar, Janssen. Card T was previously married to a subsequent employee of Takeda. Zingone F has received lecture fees from Takeda, Janssen, Norgine. The other authors declare that they have no conflicting interests.
Author Contribution
Conceptualization: Zingone F. Drafting study: Barberio B, Zingone F. Data collection: Barberio B, Baldisser F, Gubbiotti A, Massimi D, Ghisa M. Data analysis and interpretation: Zingone F, Canova C, Card T. Writing - original draft: Barberio B, Savarino EV, Zingone F. Writing - review and editing: all authors. Approval of final manuscript: all authors.
Supplementary Material
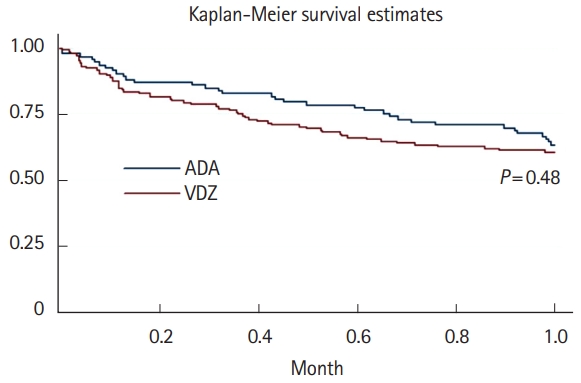
Fig.┬Ā1.

Fig.┬Ā2.
Table┬Ā1.
Table┬Ā2.
| AEs causing withdrawal | ADA (n=93) | VDZ (n=145) | ||
|---|---|---|---|---|
| Totala | 16 (17.2) | 11 (7.6) | ||
| Infusion reaction | 1 (6.2) | 2 (18.2) | ||
| ŌĆā | ┬Ę Angioedema | ŌĆā | ┬Ę Hypertensive crisis during infusion | |
| ┬Ę Post-infusion lipothymia | ||||
| Injection-site reaction | 1 (6.2) | 0 | ||
| Infection | 5 (31.2) | 3 (27.3) | ||
| ┬Ę Recurrent otitis | ┬Ę Bronchitis | |||
| ┬Ę Pyelonephritis | ┬Ę Cytomegalovirus reactivation | |||
| ┬Ę Bilateral pneumonia | ┬Ę Cough, dyspnea | |||
| ┬Ę Cough, dyspnea | ||||
| ┬Ę Herpes simplex virus reactivation | ||||
| Noninfectious extraintestinal events | 7 (43.7) | 6 (54.5) | ||
| ┬Ę Headache and joint pain | ┬Ę Joint pain worsening and psoriasis | |||
| ┬Ę Chronic fatigue syndrome | ┬Ę Psoriatic lesions | |||
| ┬Ę Dermatitis and joint pain | ┬Ę Urticaria and rash | |||
| ┬Ę Psoriatic-like rash | ┬Ę Heartbeat | |||
| ┬Ę Thrombophlebitis | ┬Ę Anxiety and panic attacks | |||
| ┬Ę Alopecia (├Ś 2) | ┬Ę Joint pain worsening | |||
| Malignancy | 2 (12.5) | 0 | ||
| ┬Ę Melanoma in situ | ||||
| ┬Ę T-lymphoproliferative disease with a high degree of malignancy | ||||
| Death | 0 | 0 | ||
Table┬Ā3.
| Variable |
Overall period |
P-value for HR | |||||
|---|---|---|---|---|---|---|---|
| No. of Aes ADA/VDZ | Rates per 100 PY with CIs in ADA | Rates per 100 PY with CIs in VDZ | Unadjusted HR (95% CI) | Adjusted HRa (95% CI) | |||
| All | 16/11 | 13.2 (8.1-21.5) | 5.3 (2.9-9.6) | 0.41 (0.19-0.89) | 0.24 (0.08-0.73) | 0.01 | |
| Disease | |||||||
| ŌĆā | Ulcerative colitis | 3/2 | 9.2 (2.9-28.6) | 2.5 (0.6-9.8) | 0.27 (0.04-1.64) | 0.43 (0.06-3.11) | 0.41 |
| CrohnŌĆÖs disease | 13/9 | 14.6 (8.5-25.2) | 7.1 (3.7-13.7) | 0.48 (0.20-1.15) | 0.15 (0.03-0.70) | 0.02 | |
| Age (yr) | |||||||
| Ōēż 35 | 1/1 | 3.1 (0.4-22.2) | 2.0 (0.3-14.1) | 0.85 (0.05-13.60) | - | - | |
| 36-54 | 12/6 | 17.3 (9.8-30.5) | 6.2 (2.8-13.9) | 0.37 (0.14-1.00) | 0.20 (0.04-0.89) | 0.35 | |
| Ōēź 55 | 3/4 | 14.9 (4.8-46.2) | 6.5 (2.4-17.4) | 0.43 (0.09-1.98) | 0.23 (0.03-1.86) | 0.17 | |
| Sex | |||||||
| Male | 7/4 | 8.7 (4.1-18.2) | 3.0 (1.1-8.1) | 0.35 (0.10-1.21) | 0.27 (0.04-1.64) | 0.15 | |
| Female | 9/7 | 22.0 (11.5-42.4) | 9.2 (4.4-19.3) | 0.45 (0.17-1.23) | 0.24 (0.05-1.03) | 0.06 | |
| Previous biological therapy | |||||||
| None | 4/1 | 6.5 (2.4-17.5) | 3.8 (0.5-27.3) | 0.59 (0.06-5.33) | 0.54 (0.05-5.18) | 0.59 | |
| 1 Biological therapy | 12/2 | 20.8 (11.8-36.7) | 2.0 (1.0-16.4) | 0.20 (0.04-0.93) | 0.15 (0.03-0.85) | 0.03 | |
| Ōēź 2 Biological therapies | 0/8 | - | 6.0 (3.0-12.1) | - | - | - | |
| Time from the last biologic therapy | |||||||
| No biologic therapies (naïve) or from more than 6 mo | 8/7 | 10.7 (5.4-21.5) | 7.2 (3.4-15.1) | 0.67 (0.24-1.87) | 0.23 (0.04-1.39) | 0.11 | |
| Ōēż 6 mo | 8/4 | 17.0 (8.5-38.0) | 3.6 (1.4-9.7) | 0.22 (0.06-0.74) | 0.26 (0.04-1.52) | 0.13 | |
| Indication to therapy | |||||||
| Active disease | 15/11 | 13.3 (8.0-22.0) | 1.8 (3.3-10.8) | 0.45 (0.20-0.99) | 0.26 (0.07-0.88) | 0.03 | |
| Post-surgery | - | - | - | - | - | - | |
| Intolerant to previous biologic therapy | 1/0 | 13.9 (1.9-89.5) | - | - | - | - | |
| Azathioprine during the year before the start of the treatment | |||||||
| No | 15/10 | 13.7 (8.3-22.7) | 5.5 (2.9-10.3) | 0.42 (0.18-0.94) | 0.26 (0.09-0.81) | 0.02 | |
| Yes | 1/1 | 8.3 (1.2-59.3) | 3.7 (0.5-26.1) | 0.64 (0.04-11.03) | - | - | |
| Steroids during the year before the start of the treatment | |||||||
| No | 13/7 | 13.2 (7.6-22.7) | 4.2 (2.0-8.9) | 0.32 (0.13-0.82) | 0.13 (0.03-0.61) | 0.01 | |
| Yes | 3/4 | 13.1 (4.2-40.6) | 9.4 (3.5-24.9) | 0.66 (0.13-3.31) | 0.64 (0.11-3.67) | 0.61 | |
| Azathioprine ongoing | |||||||
| No | 15/10 | 15.4 (9.3-25.6) | 6.0 (3.2-11.1) | 0.38 (0.17-0.86) | 0.23 (0.07-0.75) | 0.01 | |
| Yes | 1/1 | 4.1 (0.6-29.1) | 2.5 (0.3-17.7) | 0.79 (0.05-12.78) | - | - | |
| Steroid ongoing | |||||||
| No | 12/7 | 12.8 (7.3-22.6) | 5.2 (2.5-10.9) | 0.41 (0.16-1.04) | 0.22 (0.06-0.74) | 0.01 | |
| Yes | 4/4 | 14.3 (5.4-38.1) | 5.5 (2.0-14.6) | 0.39 (0.09-1.58) | 0.89 (0.06-12.21) | 0.93 | |
Table┬Ā4.
REFERENCES
- TOOLS