Physician education can minimize inappropriate steroid use in patients with inflammatory bowel disease: the ACTION study
Article information
Abstract
Background/Aims
Epidemiological data on steroid use in South Korean patients with inflammatory bowel disease (IBD) are limited. We documented the steroid use patterns in these patients, and whether physician education on appropriate steroid use affected these patterns.
Methods
ACTION was an observational cohort study conducted in adults (≥19 years) with IBD. A retrospective chart review was performed at baseline (cohort 1) and 1 year after physician training (cohort 2). Eligible cases with excessive or inappropriate steroid use were identified, along with any associated risk factors.
Results
Data were collected during May 2018-July 2019 from patients with Crohn’s disease (CD) and ulcerative colitis (UC) in cohort 1 (n=1,685) and cohort 2 (n=1,649). At baseline, 155 patients (9.2%) had received steroids within the previous 12 months, 46 (29.7%) of whom had used steroids excessively, 16 (34.8%) of these having inappropriately used excessive steroids. Although steroid exposure was similar in cohort 1 (9.2%) and cohort 2 (9.7%), the latter comprised fewer excessive steroid users (20.0% vs. 29.7%). Severe disease was associated with excessive steroid use in cases with UC, but not with CD.
Conclusions
Although, overall steroid use was relatively low in South Korean patients with IBD, one-third of steroid users used them excessively, and one-third among these used excessive steroids inappropriately. High disease activity was the main risk factor for excessive steroid use which may potentially be reduced by physician education, especially in cases with UC. Active screening to minimize excessive and inappropriate steroid use through physician education should be considered.
INTRODUCTION
Inflammatory bowel disease (IBD) is a non-infectious, chronic inflammatory disorder of the gastrointestinal tract, with Crohn’s disease (CD) and ulcerative colitis (UC) being the most dominant forms of the disease [1]. CD is characterized by discontinuous regions of inflammation that can manifest along any portion of the gastrointestinal tract [2]. Inflammation in CD is transmural and can result in pain, diarrhea, obstruction, and perianal disease [2]. Unlike CD, UC affects only the colonic mucosa [3]. Patients with UC usually present with abdominal discomfort and rectal bleeding [3].
Increasing incidence and prevalence of IBD worldwide is expected to impart a substantial socioeconomic burden on several nations [4], including South Korea, where the incidence and prevalence of IBD is one of the highest among Asian nations [5,6]. Despite increasing use of biologics such as tumor necrosis factor-α antagonists and the approval of novel therapies, the use of corticosteroids remains widespread due to the high cost of these newer treatments [7].
Corticosteroids (hereby referred to as steroids) are effective anti-inflammatory agents that can induce remission in patients with active UC [8] or CD [9]. Despite their short-term efficacy, extended steroid use has been associated with serious adverse events ranging from bone loss, risk of infection, hypertension, adrenal suppression, immunosuppression, hyperglycemia and diabetes, dyslipidemia, and dermatological and mood disorders [10]. Furthermore, prolonged treatment with steroids is ineffective in maintaining IBD remission, with systemic steroids not being associated with inducing or maintaining mucosal healing in IBD [11,12]. Steroids are also associated with greater mortality and morbidity compared with tumor necrosis factor-α inhibitors [13].
In view of the adverse impact of long-term steroid use, several medical societies advise caution in the administration of steroids in patients with IBD [14,15]. In South Korea, the IBD Study Group of the Korean Association for the Study of Intestinal Diseases (KASID) recommends that oral steroids should not be provided as front-line therapy to patients with mild-to-moderate UC [16]. These guidelines further recommend that systemic steroids may be used as first-line induction therapy for moderate-to-severe CD, but gradual dose reduction commensurate with patient response and disease severity is advised [17]. However, maintenance therapy with steroids is not recommended for patients with CD [17] or UC [16]. Prevention of corticosteroid-induced adverse events may be achieved by avoiding prolonged or repetitive use, and by switching appropriate patients to immunomodulator and/or biologic therapy [18-21].
While consequences of inappropriate steroid use are well-documented, epidemiological data describing the prevalence of steroid use in South Korean patients with IBD are limited. ACTION (A retrospective Chart review to observe The use of steroids In managing patients with inflammatory bowel disease iN South Korea) was therefore conducted, with the aim of documenting the frequency of steroid use, excessive steroid use, and inappropriate steroid use in patients with IBD across South Korea. Steroid dependency as defined by the European Crohn’s and Colitis Organisation (ECCO) [22] and the Toronto Consensus guidelines [23], was used to determine the frequency of excessive or inappropriate steroid use in eligible participants. As a secondary objective, we aimed to examine whether educating physicians in South Korea about the ECCO guidelines impacted excessive and/or inappropriate steroid use in their patients with IBD.
METHODS
1. Study Design
ACTION was a non-interventional, multicenter, observational cohort study that enrolled participants with IBD (UC or CD) across 5 IBD centers in South Korea. A retrospective chart review of patients with IBD was performed at baseline (Year 0) and 1 year after physician training (Year 1). The educational training, which commenced 1 month after baseline, comprised a medical expert who educated physicians face-to-face on appropriate steroid use, steroid refractoriness and dependency as defined by KASID [16,17], British Society of Gastroenterology [14], and ECCO [22]. Physicians received a monthly email reminder of the ECCO guidelines for 6 months following the training period. This educational intervention was directed solely at physicians treating patients with IBD. Summary of the study design is shown in Fig. 1. The details of the guidelines used to educate the physicians are provided in Supplementary Tables 1-3.
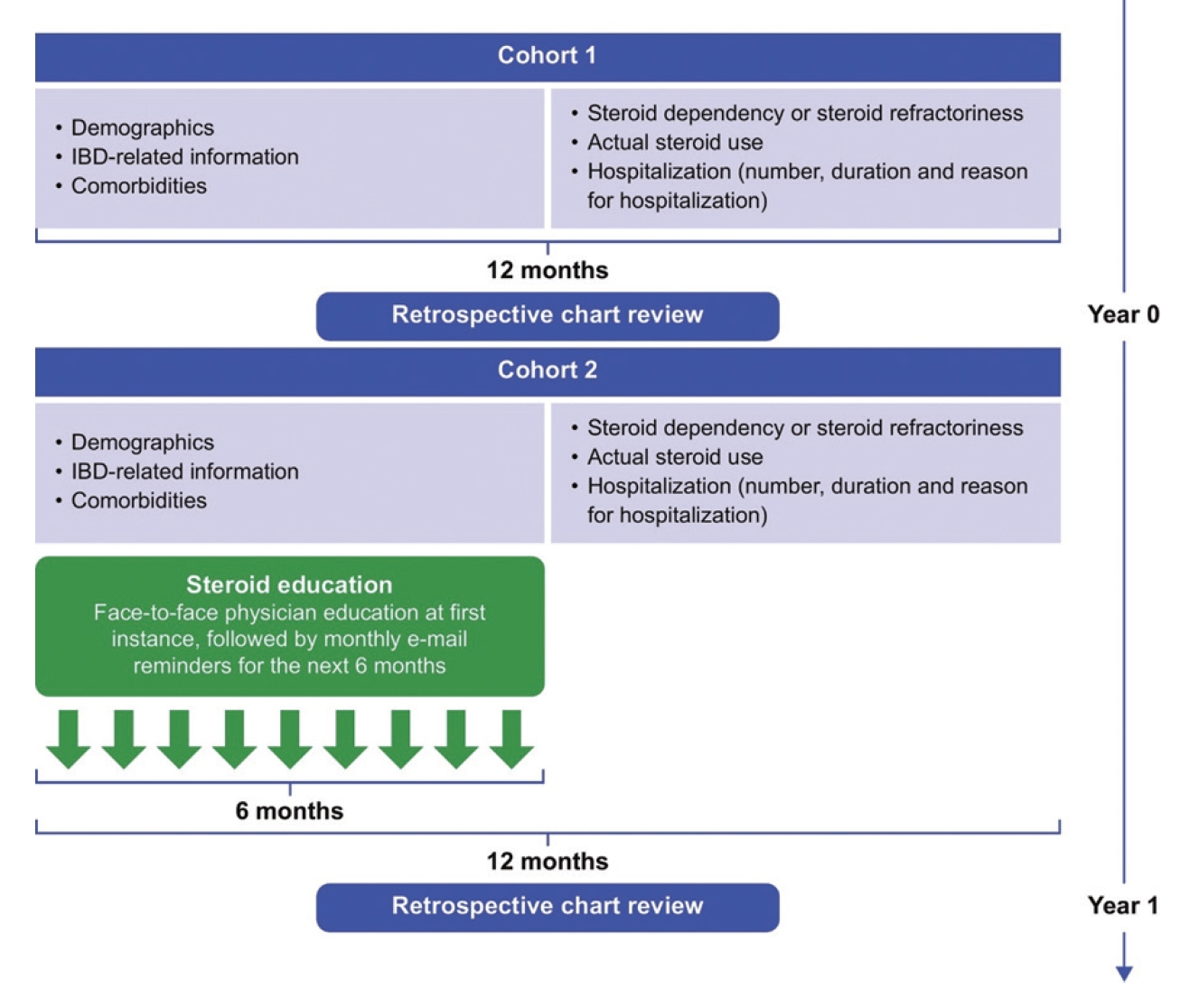
The study design of ACTION is shown in the following schematic. A retrospective chart review of patients with inflammatory bowel disease (IBD) was performed at baseline (Year 0) and 1 year after physician training (Year 1), documenting demographic information and steroid use patterns in these patients. The educational training commenced 1 month after baseline and comprised a medical expert who educated physicians face-to-face on appropriate steroid use, steroid refractoriness and dependency as defined by the Korean Association for the Study of Intestinal Diseases, British Society of Gastroenterology, and European Crohn’s and Colitis Organisation (ECCO). Physicians received a monthly email reminder of the ECCO guidelines for 6 months following the training period.
2. Participants and Site Selection
Study participants were eligible if aged ≥ 19 years and diagnosed with either UC or CD. Availability of ≥ 12 months of medical records on IBD management was required. Exclusion criterion was enrollment in an interventional study anytime during the past year prior to enrollment (Year 0) and/or within 1 year of enrollment in the current study. Study sites (Supplementary Table 4) were selected based on the following criteria: presence of a separate IBD center in the hospital, a gastroenterologist specializing in IBD practicing at the center, and a sufficiently large number of potentially eligible patients treated by IBD specialists. Participants whose data were reviewed at baseline (Year 0) comprised cohort 1 and participants whose data were reviewed 1 year after physician education (Year 1), comprised cohort 2.
3. Outcomes
The primary outcome of this study was the percentage of patients using excessive amounts of steroids within the year prior to the baseline (Year 0), with excessive steroid use defined as steroid use despite steroid dependency or refractoriness. Patients were considered to have steroid dependency if they met at least one of the following conditions defined by the ECCO and Toronto Consensus guidelines: (1) inability to reduce the dose of steroids below the equivalent dose of prednisolone 10 mg/day within 3 months of starting steroids, without recurrent active disease; (2) recurrence of symptoms within 3 months after stopping steroids; and (3) the use of 2 or more steroid courses within 1 year [22,23]. Steroid-refractory disease was defined according to the ECCO guidelines as the incidence of active disease despite receiving prednisolone up to 1 mg/kg/day for 4 weeks.
Secondary endpoints included the proportion of patients with excessive steroid use 1 year before Year 1, inappropriate steroid use which was defined as continued use of steroids despite excessive use without stepping-up medical management, at Year 0 and Year 1; risk factors of excessive or inappropriate steroid use at Year 0 and Year 1; change in the percentage of patients with excessive steroid use between Year 0 and Year 1, change in the Crohn’s Disease Activity Index (CDAI) or the partial Mayo score for UC, between Year 0 and Year 1; number of hospitalization events, duration and reason for hospitalization at Year 0 and Year 1; use of other therapies including steroids; prevalence of steroid refractoriness; and the percentage of patients with any steroid use within the preceding year at Year 0 and Year 1.
4. Data Collection
Retrospective chart reviews were conducted on 2 patient cohorts. Patients who met the enrollment criteria and visited the site approximately 1 month before baseline (Year 0) were included in cohort 1. One year after physician education (Year 1), eligible patients who visited the center within 1 month before Year 1 were included in cohort 2.
Demographic and clinical information including sex, age, comorbidities, CD or UC disease diagnosis and phenotype were recorded for both cohorts. CDAI and partial Mayo scores were determined at Year 0 and Year 1, as well as 1 year prior to study visit. Disease activity status was categorized as remission (CDAI < 150 or partial Mayo score 0–1); mild (CDAI ≥ 150 to < 220 or partial Mayo score 2–4); moderate (CDAI ≥ 220 to < 450 or partial Mayo score 5–6); and severe (CDAI ≥ 450 or partial Mayo score 7–9). Surgical history related to IBD was coded according to the Medical Dictionary for Regulatory Activities (MedDRA, version 22.0) and categorized by System Organ Class and Preferred Term. Steroid and other medications used to treat IBD during the 12 months prior to data collection in both cohorts were classified according to the Anatomical Therapeutic Chemical 2019 classification.
Charts were automatically sorted by each center’s electronic medical record system and were not determined by the investigator.
5. Sample Size Calculation and Bias
Patients were recruited from 5 centers across the 5 geographic regions of South Korea, in order to ensure enrollment of a representative sample of patients with IBD. It was calculated that a sample size of 1,932 patients per cohort would result in a 95% confidence interval (CI) with a precision of 1.5%, based on a recent multicenter audit of excessive steroid use conducted in the United Kingdom (UK), in which the prevalence of excessive steroid use was estimated to be approximately 14% among the 32,000 patients with IBD who were evaluated [24]. Heterogeneity < 5% was expected between cohorts 1 and 2 due to patient dropout and recruitment of new patients between Year 0 and Year 1. Patients in cohorts 1 and 2 were managed by the same physicians to minimize bias caused by different clinical practice patterns, although it was likely that physicians who participated in Year 0 may no longer have been at the center in Year 1.
6. Statistical Analysis
Continuous variables were summarized descriptively (number of patients, mean and standard deviation [SD]), and categorical variables were summarized using frequencies and percentages. No imputation for missing data was used. Categorical variables between the 2 cohorts were compared by the chi-square or Fisher exact test.
Change in the number and percentage of patients with excessive steroid use or inappropriate steroid use during the study were summarized using shift tables. Changes in excessive or inappropriate steroid use were evaluated using chi-square test (for entire cohorts) and McNemar test (for cases common in both cohorts). Multivariate logistic regression analyses were performed to determine the risk factors for excessive steroid use. Fisher exact test was used to evaluate the relationship between excessive steroid use and disease activity status. A generalized estimation equation analysis was used to confirm the effectiveness of the medical education provided. Statistical significance was set at P<0.05. Analyses were performed using the statistical software SAS, version 9.3 (SAS Institute, Cary, NC, USA).
7. Ethics Statement
This study was registered at each participating study site and adhered to the guidelines for Good Pharmacoepidemiology Practices for non-interventional studies and national legislation. Institutional review board approval was obtained from each participating center, including Kangbuk Samsung Hospital (IRB No. KBSMC2018-01-047). Informed consent from the study participants was not required as this was a retrospective study involving review of medical charts of patients from the different participating institutes.
RESULTS
1. Participant Characteristics
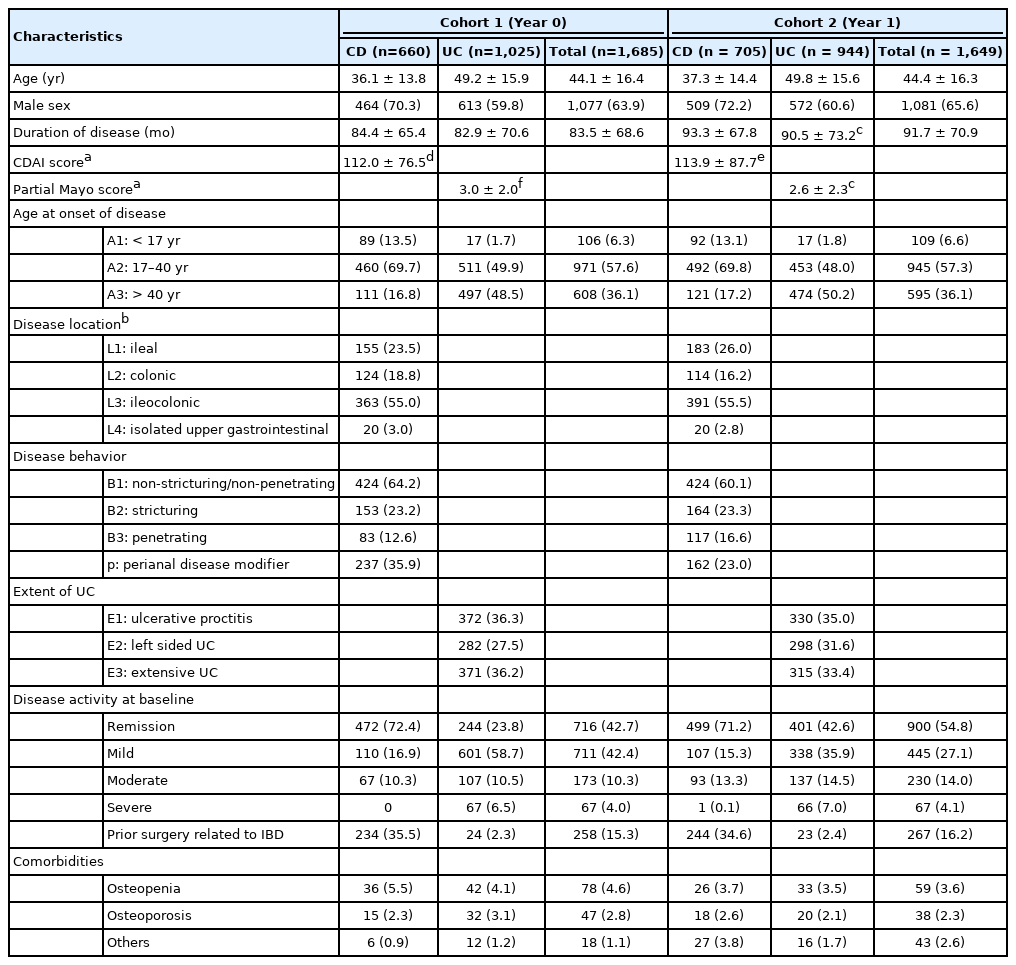
The study was conducted from May 14, 2018 to July 18, 2019. Data were collected at Year 0 from a total of 1,685 participants in cohort 1 (660 CD and 1,025 UC). The impact of physician education on steroid use was evaluated from the data collected from 1,649 patients in cohort 2 at Year 1 (705 CD and 944 UC).
Baseline characteristics were generally similar between the 2 cohorts (Table 1). The mean ± SD age in cohort 1 was 44.1 ± 16.4 years and 63.9% (n = 1,077) of the participants were male, while the mean ± SD age in cohort 2 was 44.4 ± 16.3 years and 65.6% (n = 1,081) of the participants were male. The mean ± SD duration of disease in cohort 1 was 83.5 ± 68.6 months and 91.7 ± 70.9 months in cohort 2. Mean ± SD CDAI scores in participants with CD (cohort 1, 112.0 ± 76.5; cohort 2, 113.9 ± 87.7) and partial Mayo scores in participants with UC (cohort 1, 3.0 ± 2.0; cohort 2, 2.6 ± 2.3) at 12 months prior to the year of analysis were similar between the 2 cohorts.
Most participants diagnosed with CD had ileocolonic (L3) involvement (cohort 1, n = 363 [55.0%]; cohort 2, n = 391 [55.5%]) and non-stricturing non-penetrating (B1) behavior (cohort 1, n = 424 [64.2%]; cohort 2, n = 424 [60.1%]). The most common type of UC in both cohorts was proctitis (E1; cohort 1, n = 372 [36.3%]; cohort 2, n = 330 [35.0%]) followed by extensive UC (E3; cohort 1, n = 371 [36.2%]; cohort 2, n = 315 [33.4%]). At baseline, 4.0% and 4.1% of the participants had severe disease activity in cohort 1 and cohort 2, respectively. Rates of prior surgery related to IBD (Table 1, Supplementary Tables 5 and 6) and the presence of comorbidities such as osteopenia and osteoporosis, were similar between the 2 cohorts.
2. The Pattern of Steroid Exposure, Excessive Steroid Use and Inappropriate Steroid Use in Cohorts 1 and 2
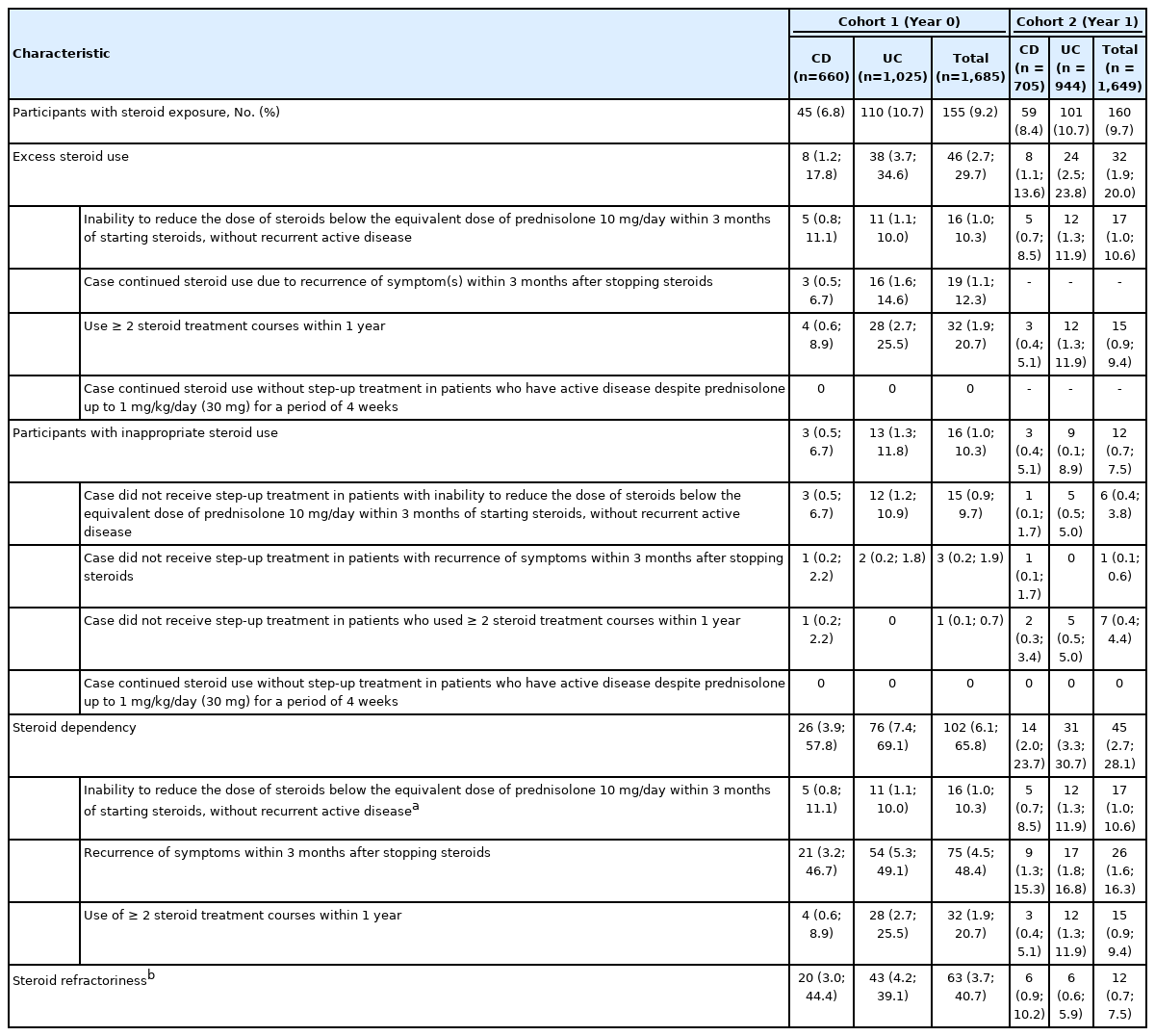
At baseline (cohort 1), the overall proportion of patients exposed to steroids in the 12 months prior to measurement was 9.2% (n = 155), with excessive steroid use being observed in 2.7% (n = 46) of participants, and a frequency of 29.7% among participants with steroid exposure (Table 2, Supplementary Table 7). Overall steroid exposure was not significantly different in cohort 2 following medical education of physicians (n = 160, 9.7%); however, excessive steroid use was reported in fewer participants than in cohort 1 (n = 32; 1.9%), which was 20.0% of all steroid users. Inappropriate steroid use, defined as the continued use of excess steroid without step-up in treatment was observed in 1.0 % (n = 16) and 0.7% (n = 12) of all participants in cohort 1 and cohort 2, respectively, at a frequency of 10.3% and 7.5% among steroid users, and 34.8% and 37.5% among excessive steroid users.
3. Patterns of Steroid Use According to Disease Type
Patients with UC showed a higher frequency of overall steroid exposure (10.7% vs. 6.8%) in cohort 1 (Table 2), compared with patients with CD. In cohort 1, among participants with steroid exposure, those with UC showed a higher rate of steroid dependency compared with participants with CD (69.1% vs. 57.8%) as well as a higher frequency of excessive steroid use (34.6% vs. 17.8%). While overall steroid exposure in cohort 2 was similar to that in cohort 1, steroid dependency among steroid users was significantly reduced in both disease types, with a reduction from 57.8% to 23.7% observed in patients with CD, and from 69.1% to 30.7% in patients with UC. Excessive steroid use was also reduced after physician education with 13.6% of steroid users with CD and 23.8% of steroid users with UC, using excessive amounts of steroids.
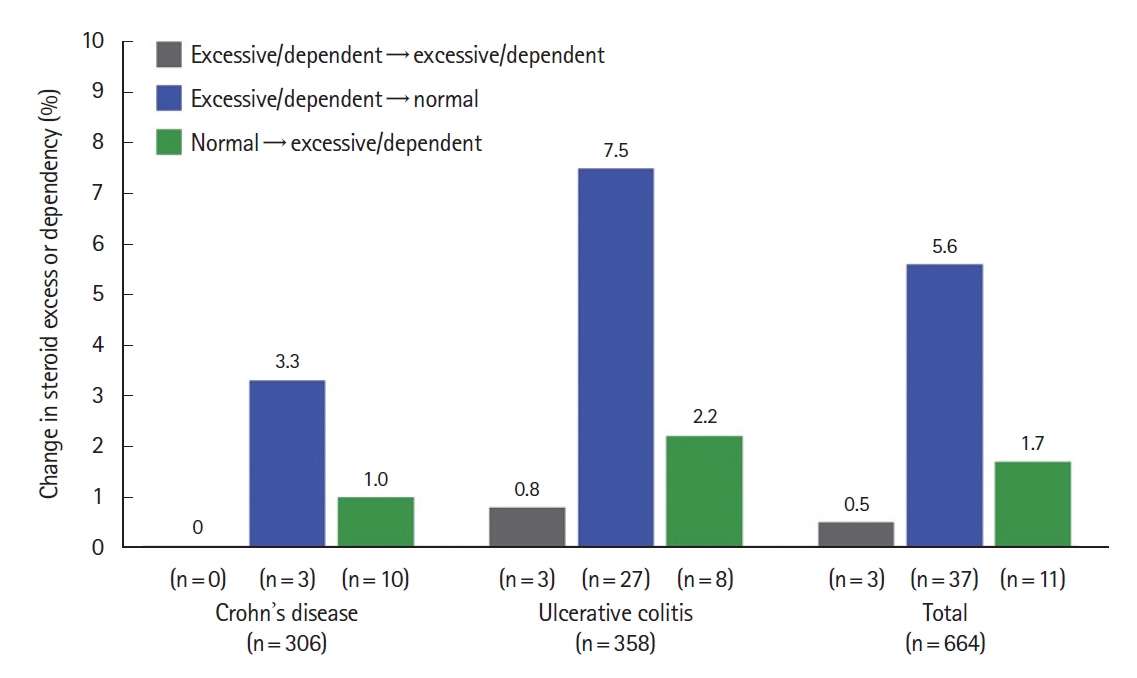
Although the effect of physician education on steroid use patterns is evident from the decrease in excessive steroid use and inappropriate steroid use in cohort 2 compared with cohort 1, a heterogeneity of 60% between the cohorts was recorded. Thus, a subset of the 664 matched-cohort patients who participated in both cohorts were evaluated for a change in excessive steroid use or steroid dependency. Overall, 5.6% of participants experienced a shift from excessive steroid use or steroid dependency to non-excessive steroid use after physician medical education (P<0.0002 according to the McNemar test) (Fig. 2, Supplementary Table 8). Patients diagnosed with UC showed a greater change from excessive to non-excessive steroid use than patients with CD, at 7.5% and 3.3%, respectively (Fig. 2). Fewer participants had a shift from non-excessive to excessive steroid use during the study (1.7%).
4. Risk Factors Associated with Excess Steroid Use in Cohorts 1 and 2
Disease-specific parameter CDAI in patients with CD, was not a risk factor that significantly impacted excessive steroid use since the odds ratio of the association of CDAI score in cohort 1 and cohort 2 was 1.00 (95% CI, 1.00–1.01; P=0.0345) and 1.01 (95% CI, 1.00–1.01; P<0.0001) respectively (Supplementary Table 9). However, the partial Mayo score in patients with UC, was a risk factor that significantly impacted excessive steroid use in both cohorts (Supplementary Table 10) with an odds ratio of 1.13 (95% CI, 1.01–1.27; P=0.0302) in cohort 1 and 1.19 (95% CI, 1.04–1.37; P=0.0133) in cohort 2, respectively. Disease activity impacts excess steroid use only in patients with UC but not CD, as observed from the P-values obtained when participants with different disease activity (remission, mild, moderate and severe) were separately analyzed (Supplementary Table 11).
Since CDAI and partial Mayo score were risk factors for excessive steroid use based on the multivariable logistic regression model analysis, we additionally evaluated the relationship between excessive steroid use and disease activity status using the Fisher’s exact test. The correlation between excessive steroid use and disease activity status was not statistically significant in patients with CD, but it was significant in patients with UC in both cohorts (P>0.05 for both cohorts in CD, P=0.0161 and P=0.0391 for cohorts 1 and 2, respectively in UC), implying that disease severity in UC was a risk factor for excessive steroid use.
5. Frequency of Steroid Dependency and Steroid Refractoriness in All Cohorts
In cohort 1, 6.1% of participants (n = 102) met the criteria for steroid dependency, as defined by ECCO and the Toronto Consensus guidelines (Table 2), with 2.7% (n = 45) of all participants in cohort 2 qualifying as steroid dependent. The frequency of steroid dependency among steroid users was 65.8% and 28.1% in cohort 1 and cohort 2, respectively (Table 2). The rate of steroid refractoriness at baseline was 3.7% (n = 63), with a frequency of 40.7% among steroid users, which was reduced to 0.7% (n = 12) in cohort 2, amounting to 7.5% among steroid users.
6. The Impact of Medical Education on the Pattern of Steroid Exposure, Excessive Steroid Use and Inappropriate Steroid Use
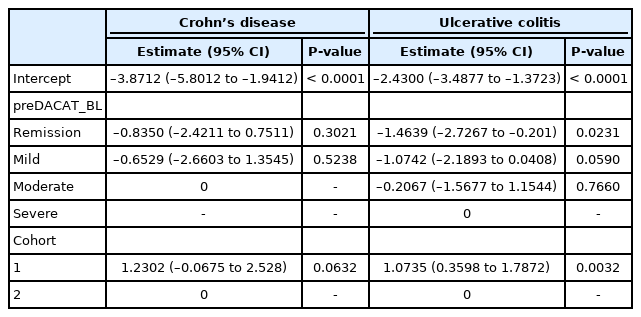
Due to the heterogeneity between cohorts 1 and 2, we conducted a generalized estimation equation analysis to confirm the effectiveness of medical education only for patients in both cohorts (n = 664) (Table 3). When the disease activity at 1 year before baseline (Year 1) was a fixed effect, there was no difference between cohorts 1 and 2 in excessive steroid use in CD (P=0.0632). On the other hand, in UC, excessive steroid use in cohort 1 is higher than that in cohort 2 (P=0.0032). This suggests the possibility that education will be effective in reducing excessive steroid use. In UC, the risk of excessive steroid use in the following 2 years after education is lesser if the disease activity is remission, compared with if it is severe (P=0.023).
7. The Impact of Medical Education on Disease Activity, Hospitalization, and Medication Use
A greater proportion of participants in cohort 2 achieved remission (n = 1,166, 71.0%) compared with cohort 1 (n = 861, 51.4%) (Table 4). More participants in cohort 1 had a correspondingly higher frequency of mild and moderate disease when compared with participants in cohort 2. The rate of severe disease was similar in both cohorts (0.7% and 0.8% in cohorts 1 and 2). A higher frequency of disease remission was observed among patients with CD (79.0% and 79.6% in cohorts 1 and 2) compared with those with UC (33.8% and 64.5% in cohorts 1 and 2). The mean ± SD CDAI score in participants with CD, was also lower in cohort 2 than that in cohort 1 (102.1 ± 71.1 vs. 95.4 ± 74.3). Among patients with UC, the mean ± SD partial Mayo score was lowered in cohort 2 (2.4 ± 1.7 vs. 1.5 ± 1.6).
Hospitalization events decreased from 10.9% (n = 184) in cohort 1 to 7.8% (n = 129) in cohort 2 (Table 4). However, the median duration of hospitalization was longer in cohort 2 (8 days), than in cohort 1 (6 days). Surgery was a major contributor to hospitalization in both cohort 1 (n = 58, 3.4%) and cohort 2 (n = 37, 2.2%). However, the rate of infection contributing to hospitalization was higher in cohort 2 (n = 15, 0.91%) than in cohort 1 (n = 12, 0.71%).
DISCUSSION
The ACTION study explored the impact of physician education on the frequency of steroid use in South Korean patients with IBD as a means to manage their disease. While the baseline overall steroid use was reasonably low at 9.2%, overall steroid exposure was not significantly altered following 1 year of physician education in accordance with definition of steroid overuse or dependency according to international guidelines. However, the frequency of excessive steroid use was significantly reduced from 29.7% in cohort 1 before physician education, to 20% in cohort 2. Dependency on steroids was also significantly reduced following 1 year of physician education from 65.8% to 28.1% of steroid users, and steroid refractoriness decreased from 40.7% to 7.5% of steroid users in this study.
Disease subtype is a key determinant of baseline steroid use, with patients diagnosed with UC being more prone to excessive steroid use or dependence, compared with patients diagnosed with CD. The severity of disease is also a contributing factor to excessive steroid use, with CDAI score in patients with CD and partial Mayo score in patients with UC, being risk factors associated with steroid overuse (odds ratio, 1.01–1.19). This trend is more prominent in UC as determined from further analysis using Fisher exact test. Disease remission was more frequent in cohort 2, and treatment burden was also significantly reduced with fewer patients requiring hospitalization in cohort 2.
Treatment goals in IBD are to eliminate symptoms of active disease, minimize disease-related complications, and improve patient quality of life [25,26]. While steroids remain the foundation of induction therapy in patients with active UC and CD, their adverse effects and poor efficacy in maintaining remission limits their prolonged use in IBD [25]. Indeed, South Korean guidelines have recommended that steroid administration in patients with severe CD be tapered off after 8 weeks, in order to balance the risk of side effects and early relapse [17]. For patients with moderate-to-severe UC, local guidelines recommend 30–40 mg/day steroids for inducing remission, with dose reduction to 2.5–20 mg/week depending on clinical improvement [16]. These recommendations are in accordance with ECCO guidelines [22].
This study had fewer patients (~10%) receiving steroids in the year before baseline measurement than a similar study conducted in the UK (~30%) [24], which may reflect the differences in clinical practice in the 2 countries. The UK study identified that steroids may have been prescribed by primary care physicians who may not be accustomed to or be aware of appropriate strategies to manage IBD [24]. In addition, since patients in our study were required to have available at least 12 months of medical records, patients with new-onset IBD who were prescribed steroids for induction therapy may have been underrepresented, leading to an underestimation of the overall prevalence of steroid use. However, among steroid users, more patients in our study had steroid dependency (65.8%) at baseline than the UK study (49.6%), suggesting the need for re-evaluation of steroid administration in South Korean patients with IBD.
The effectiveness of training by a medical expert and the monthly reminders on guidelines for appropriate steroid use was demonstrated by the reduced rates of excessive steroid use and steroid dependence. The improved remission rates and decreased frequency of hospitalizations in cohort 2 may suggest that reducing excess steroid use or steroid dependency potentially offers clinical improvements in patients with IBD. Participants whose physicians received medical training saw an increase in the prescription of immunomodulating agents which have demonstrable efficacy in IBD in case of steroid dependency [16,17], and the use of these agents instead of steroids may have contributed to improved clinical outcomes following physician education. However, the high remission rates in cohort 2 may be a consequence of selecting more patients with controlled disease. Hence, further studies investigating the impact of reducing excessive steroid use and steroid dependence on patient outcomes are required.
The observational design of this study provided an overview of steroid use that reflects real-world clinical practice. Available data on steroid use among patients in South Korea were limited. Therefore, results from this study offer useful insights in further optimizing IBD management.
However, the observational nature of this study does not account for any association of the duration of physician education and its impact on excessive steroid use with confounding variables and bias. Also, while a 5% heterogeneity between cohort 1 and cohort 2 was expected, a variability of 60% between the subgroups precludes analysis of a definitive impact of physician education on steroid use management within the same patient population. To complement this, we analyzed the effects of education only on patients who belonged to both cohorts.
To the best of our knowledge, this is the first study to assess the patterns of steroid use in South Korean patients with IBD. The overall steroid use was relatively low in South Korean patients with IBD. However, one-third of steroid users used excessive steroids, and one-third of these patients were inappropriate excessive steroid users. High disease activity was the main risk factor for excessive steroid use and educating physicians on the latest steroid use guidelines may reduce excessive steroid use, especially in UC. Therefore, active screening for excessive steroid use and minimizing inappropriate steroid use through physician education is an important strategy in ensuring more effective disease management in patients with IBD in South Korea.
Notes
Funding Source
The study was supported by AbbVie. Due to the non-interventional nature of the study, no drugs were supplied or funded. AbbVie was responsible for the study design, interpretation of data, reviewing and approving of the publication.
Conflict of Interest
Teng D is an employee of AbbVie, Inc. and holds company stock options. All other authors disclose no conflicts of interest.
Park Y, Moon HS, and Park DI are editorial board members of the journal but were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Data Availability Statement
Not applicable.
Author Contribution
Conceptualization: Park DI. Methodology: Park Y, Choi CH, Kim HS, Moon HS, Park DI. Software: Park Y, Choi CH, Kim HS, Moon HS, Kim DH, Kim JJ, Teng D, Park DI. Validation: Park DI. Formal analysis: Park Y, Choi CH, Kim HS, Moon HS, Park DI. Investigation: Park Y, Choi CH, Kim HS, Moon HS, Park DI. Resources: Park Y, Choi CH, Kim HS, Moon HS, Park DI. Data curation: Park Y. Writing - original draft: Park Y. Writing-review & editing: Park Y, Choi CH, Kim HS, Moon HS, Park DI. Visualization: Park Y. Supervision: Park DI. Project administration: Park Y, Park DI, Kim DH, Kim JJ, Teng D. Funding acquisition: Park Y, Park DI. All authors have approved the final version of the manuscript.
Others
We would like to thank the investigators and study teams, as well as the patients and their families for their participation in the study. We also thank the hospitals that participated in the study.
Medical writing support, under the direction of the authors, was provided by Isuru Wijesoma, PhD, of MediTech Media Asia Pacific, Singapore, funded by AbbVie in accordance with Good Publication Practice (GPP3) guidelines.
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Table 1. ECCO Guidelines Used to Educate Physicians for the Management of Steroid Use in Patients with CD
ir-2021-00125-suppl1.pdfSupplementary Table 2. ECCO Guidelines Used to Educate Physicians for the Management of Steroid Use in Patients with UC
ir-2021-00125-suppl2.pdfSupplementary Table 3. Guidelines per the British Society of Gastroenterology and the Toronto Consensus Guidelines for the Management of IBD Used to Educate Physicians for the Management of Steroid Use in Patients with UC
ir-2021-00125-suppl3.pdfSupplementary Table 4. Patient Disposition by Study Site in All Cohorts
ir-2021-00125-suppl4.pdfSupplementary Table 5. Surgical History Related to IBD in Cohort 1 (Year 0)
ir-2021-00125-suppl5.pdfSupplementary Table 6. Surgical History Related to IBD in Cohort 2 (Year 1)
ir-2021-00125-suppl6.pdfSupplementary Table 7. Extent of Steroid Exposure in All Cohorts
ir-2021-00125-suppl7.pdfSupplementary Table 8. McNemar Test and Chi-Square Test for Excessive Steroid Use in All Patients Between Year 0 and Year 1
ir-2021-00125-suppl8.pdfSupplementary Table 9. Risk Factors Affecting Excessive Steroid Use or Inappropriate Steroid Use in Crohn’s Disease in All Cohorts
ir-2021-00125-suppl9.pdfSupplementary Table 10. Risk Factors Affecting Excessive Steroid Use or Inappropriate Steroid Use in Ulcerative Colitis in All Cohorts
ir-2021-00125-suppl10.pdfSupplementary Table 11. Patients with Excessive Steroid Use According to Disease Activity in All Cohorts
ir-2021-00125-suppl11.pdf