Real-world effectiveness and safety of ustekinumab induction therapy for Korean patients with Crohn’s disease: a KASID prospective multicenter study
Article information
Abstract
Background/Aims
We investigated the real-world effectiveness and safety of ustekinumab (UST) as induction treatment for Koreans with Crohn’s disease (CD).
Methods
CD patients who started UST were prospectively enrolled from 4 hospitals in Korea. All enrolled patients received intravenous UST infusion at week 0 and subcutaneous UST injection at week 8. Clinical outcomes were assessed using Crohn’s Disease Activity Index (CDAI) scores at weeks 8 and 20 among patients with active disease (CDAI ≥150) at baseline. Clinical remission was defined as a CDAI <150, and clinical response was defined as a reduction in CDAI ≥70 points from baseline. Safety and factors associated with clinical remission at week 20 were also analyzed.
Results
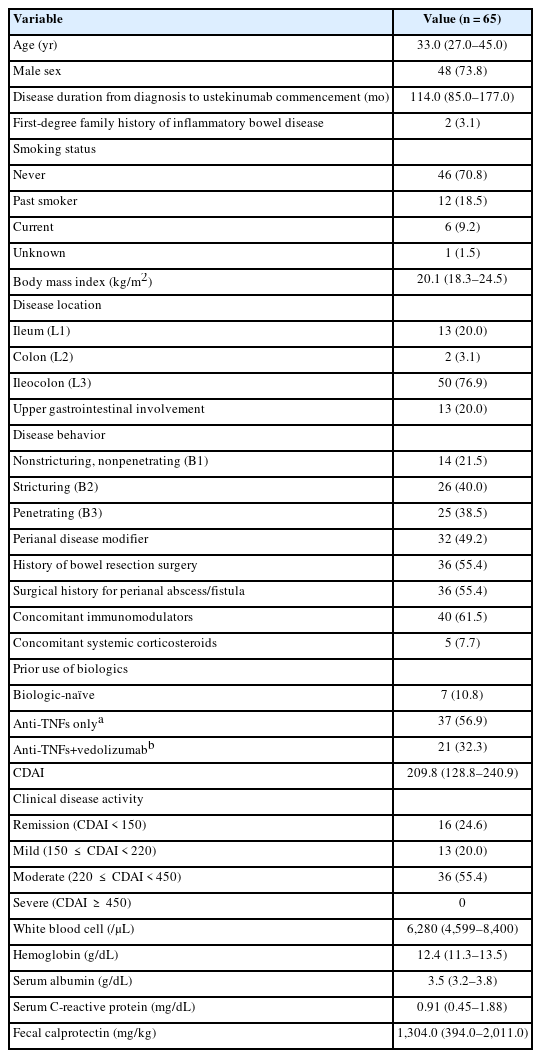
Sixty-five patients were enrolled between January 2019 and December 2020. Among 49 patients with active disease at baseline (CDAI ≥150), clinical remission and clinical response at week 8 were achieved in 26 (53.1%) and 30 (61.2%) patients, respectively. At week 20, 27 (55.1%) and 35 (71.4%) patients achieved clinical remission and clinical response, respectively. Twenty-seven patients (41.5%) experienced adverse events, with serious adverse events in 3 patients (4.6%). One patient (1.5%) stopped UST therapy due to poor response. Underweight (body mass index <18.5 kg/m2) (odds ratio [OR], 0.085; 95% confidence interval [CI], 0.014–0.498; P=0.006) and elevated C-reactive protein at baseline (OR, 0.133; 95% CI, 0.022–0.823; P=0.030) were inversely associated with clinical remission at week 20.
Conclusions
UST was effective and well-tolerated as induction therapy for Korean patients with CD.
INTRODUCTION
Although medical treatment of Crohn’s disease (CD) has significantly improved after the introduction of anti-tumor necrosis factor (TNF) agents, approximately one-fifth of patients do not initially respond to anti-TNF agents (primary nonresponse), and 23% to 46% of patients eventually lose their responses to anti-TNF agents (secondary loss of response) [1,2]. Moreover, the response rate to second- or third-line anti-TNFs has been reported to be lower among anti-TNF-failed patients with inflammatory bowel disease (IBD) than among biologic-naïve patients, and a lack of response necessitates switching to drugs with mechanisms of action different from those of anti-TNFs [3,4].
Ustekinumab (UST) is a fully human immunoglobin G1 monoclonal antibody that blocks the p40 subunit of interleukin (IL)-12 and IL-23 [5]. UST was first approved as a treatment for psoriasis [6]. IL-12 and IL-23, which modulate T-helper (Th) 1 and Th 17 cells, are key proinflammatory cytokines in the pathogenesis of CD [7,8]. Therefore, UST was expected to be efficacious in the treatment of CD by blocking the inflammatory cascade mediated by IL-12 and IL-23. UST has been proven to be efficacious as induction and maintenance therapy for patients with moderate to severe CD in randomized controlled trials [9-11]. UST induction therapy (6 mg/kg) has been shown to yield clinical responses in 38% to 57% of patients and clinical remission in 21% to 40% of patients with CD at week 8 [11]. Additionally, UST induction therapy has been shown to significantly reduce C-reactive protein (CRP) and fecal calprotectin levels [11]. UST has since been associated with clinical benefits as an induction therapy for patients with CD in several multicenter studies from Europe and North America [12-21]. In addition to the clinical response, it has also been shown to be effective for inducing short-term biomarker and/or endoscopic responses [12,14,15,17,18,21]. In a pooled analysis of phase 2 and 3 placebo-controlled trials for patients with IBD, the UST treatment group did not show any differences in terms of development of adverse events (AEs), serious AEs (SAEs), infections, serious infections, and malignancies excluding nonmelanoma skin cancer compared with the placebo group through 1 year [22].
However, most studies on UST treatment for CD have been performed in the Western world; therefore, real-world data on the clinical benefits of UST for Asian patients with CD are still lacking [23-25]. As the incidence and prevalence of IBD in Asian countries have been increasing during the last few decades [26-29], different genetic and phenotypic characteristics have been reported among Asian IBD patients compared with Western IBD patients [30-32]. Unlike with Caucasian patients, there have been conflicting reports on the association between genetic polymorphism of IL-23 receptors (IL23Rs) and CD susceptibility among East Asian patients [33,34]. Therefore, the real-world effectiveness and safety of UST among Asian patients must be evaluated and compared with Western data. We investigated the clinical effectiveness and safety profile of UST induction therapy for Korean patients with CD.
METHODS
This was a prospective, observational, multicenter study. Patients with CD who received UST induction therapy between January 2019 and December 2020 at any of 4 participating centers in Korea were prospectively enrolled in the Korean Association for the Study of Intestinal Diseases UST Registry. At week 0, UST was intravenously infused (260 mg for patients with body weight ≤ 55 kg, 390 mg for patients with body weight > 55 kg but ≤ 85 kg, and 520 mg for patients with body weight > 85 kg), followed by subcutaneous injection of UST 90 mg at week 8. Concomitant therapy with 5-acetylsalicylic acids, immunomodulators, or corticosteroids was permitted. The study protocol was approved by the institutional review board of each participating center, including Asan Medical Center (IRB No. 2018-1388). All study participants provided written informed consent prior to study enrollment.
1. Data Collection
We collected demographic characteristics and CD disease characteristics: age, sex, duration of disease, family history of IBD in first-degree relatives, smoking status, body mass index (BMI), history of bowel resection, previous surgical history for perianal abscess/fistula, concomitant medications, prior use of biologics, Montreal disease location, and Montreal disease behavior [35]. We also collected Crohn’s Disease Activity Index (CDAI) scores and laboratory data, including CRP, serum albumin, and fecal calprotectin levels at baseline (week 0), week 8, and week 20.
2. Outcomes
Clinical outcomes were assessed using CDAI scores at weeks 8 and 20 among patients with active disease (CDAI score ≥ 150) at baseline. Clinical outcomes included the achievement of clinical remission or clinical response. The co-primary outcomes were clinical remission after UST induction therapy (CDAI score < 150 at week 20) or clinical response (reduction in CDAI score ≥ 70 points from baseline [CDAI-70]) at week 20.
Secondary outcomes included corticosteroid-free clinical remission and response, which were defined as clinical remission or response without the need for concomitant systemic corticosteroids (budesonide, prednisone, prednisolone, or methylprednisolone). Changes in biochemical markers, such as serum albumin, serum CRP, and fecal calprotectin levels, were also analyzed. All patients were asked about AEs, including infusion reactions, injection site reactions, exacerbation of CD, infections, hospitalizations, any surgery related to CD, and any potential AEs at regular or unexpected visits. The AEs that resulted in CD-related hospitalization, CD-related bowel surgery, or death were considered SAEs. CD exacerbation was defined as an increase in CDAI ≥ 100 points from baseline, an addition or dose escalation of CD-related drugs, CD-related bowel surgery, or CD-related hospitalization.
3. Statistical Analysis
Patients who received at least 1 dose of UST were included in the effectiveness and safety analyses. Therefore, those who stopped UST or who underwent CD-related bowel surgery before each clinical evaluation were considered to have not achieved clinical effectiveness outcomes. Categorical variables and continuous variables are expressed as numbers with percentages and medians with interquartile ranges (IQRs), respectively. Linear mixed modeling was used to evaluate continuous measurements of CDAI scores and laboratory values, including serum CRP, serum albumin, and fecal calprotectin at weeks 0, 8, and 20. Factors associated with clinical remission and clinical response at week 20 were identified using univariable and multivariable logistic regression analysis. Multivariable analysis was performed using the backward elimination method, including variables with P<0.1 in the univariable analyses. Statistical analyses were performed using SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY, USA).
RESULTS
1. Patient Characteristics
A total of 65 patients who received UST induction therapy were enrolled. The baseline characteristics of the study participants are summarized in Table 1. Forty-eight patients (73.8%) were men, and the median age at the commencement of UST was 33 years (IQR, 27–45 years). The median disease duration from diagnosis to the first administration of UST was 114 months (IQR, 85–177 months). Fifty-one patients (78.5%) had stricturing or penetrating behavior. Thirty-two patients (49.2%) had perianal disease modifiers, and there were 36 patients (55.4%) each with a history of bowel resection and a surgical history for perianal abscess/fistula. There were 40 patients (61.5%) who were taking concomitant immunomodulators and 5 patients (7.7%) taking concomitant corticosteroids with UST, respectively. Only 7 patients (10.8%) were biologic-naïve, and 10 patients (15.3%) had received 3 biologics. The median CDAI score at baseline was 209.8 (IQR, 128.8–240.9).
2. Clinical Outcomes during UST Induction Therapy
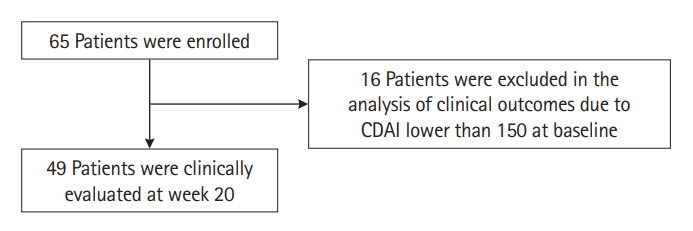
Among 49 patients who had a CDAI of 150 or higher at baseline (Fig. 1), clinical remission and clinical response at week 8 were achieved in 26 (53.1%) and 30 (61.2%) patients, respectively. At week 20, 27 (55.1%) and 35 (71.4%) patients achieved clinical remission and clinical response, respectively (Fig. 2). Corticosteroid-free clinical remission/response were achieved in 25 (51.0%)/26 (53.1%) patients at week 8 and 29 (59.2%)/34 (69.4%) patients at week 20, respectively (Fig. 2). Among 49 patients, the median CDAI score at baseline was 238.0 (IQR, 214.8–257.0), and the median CDAI scores at week 8 and week 20 were 142.4 (IQR, 90.4–193.1) and 129.5 (IQR, 90.65– 190.11), respectively (both P<0.001) (Fig. 3A). Out of 49 patients, 44 (89.8%) were biologic-exposed, and 5 (10.2%) were naïve to biologics. Among 44 biologic-exposed patients, clinical remission/clinical response rates were 47.7%/56.8% at week 8 and 50.0%/68.2% at week 20, respectively. Corticosteroid-free clinical remission/response rates among those 44 patients were 45.5%/54.5% at week 8 and 47.7%/65.9% at week 20, respectively. Among 5 biologic-naïve patients, clinical remission and response rates at weeks 8 and 20 were all 100%. Corticosteroid-free clinical remission/response rates for those 5 patients were also all 100% at weeks 8 and 20.

Clinical outcomes at week 8 and week 20 among 49 patients with Crohn’s Disease Activity Index score 150 or over at baseline.
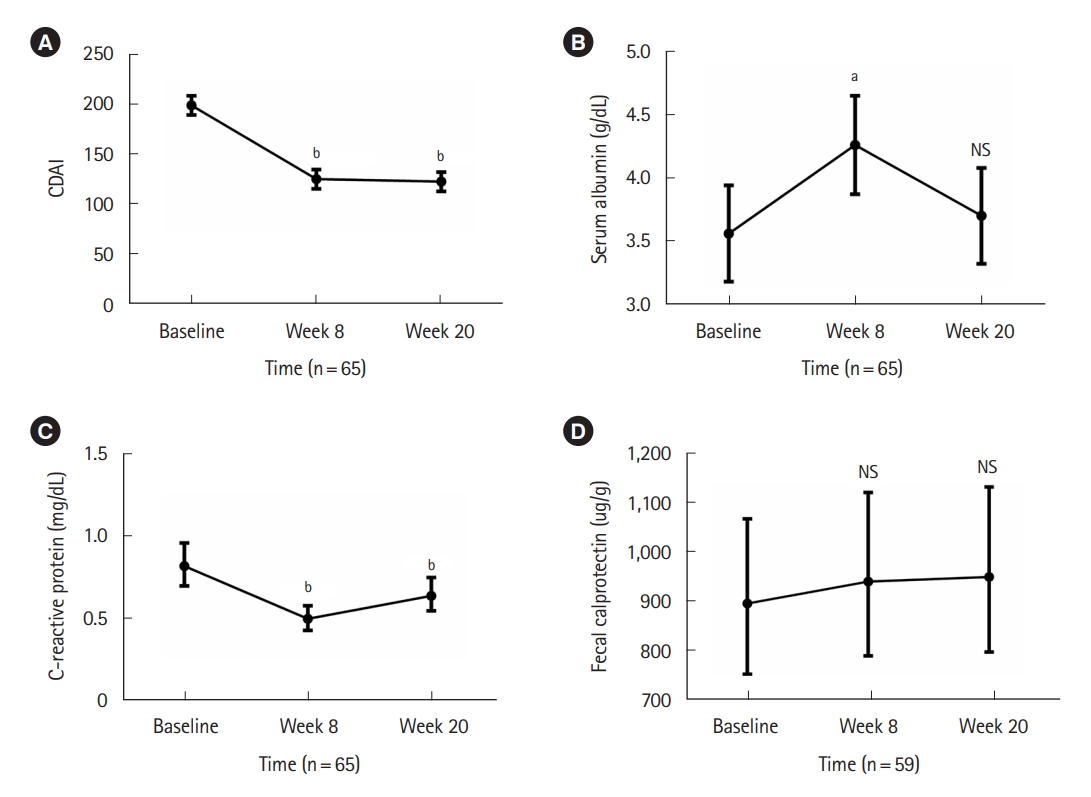
Changes in Crohn’s Disease Activity Index (CDAI) and laboratory values over time. CDAI (A), serum albumin level (B), serum C-reactive protein level (C), and fecal calprotectin level (D) are expressed in means±standard error of the means. The levels of C-reactive protein and fecal calprotectin were log-transformed, analyzed, and back-transformed. aP<0.05, bP<0.001. NS, not significant.
3. Changes in Biochemical Markers
Relative to baseline, the mean serum albumin level showed a significantly higher level (P<0.05) at week 8 but not at week 20 (Fig. 3B). In the case of CRP, the mean levels at week 8 and week 20 were both significantly lower (P<0.001) compared with baseline (Fig. 3C). In contrast, the mean fecal calprotectin level (Fig. 3D) did not show significant changes during induction therapy.
4. Predictors of Clinical Remission and Clinical Response at Week 20
In the multivariable analysis, underweight (BMI < 18.5 kg/m2) (odds ratio [OR], 0.085; 95% confidence interval [CI], 0.014–0.498; P=0.006) and elevated CRP (≥ 0.6 mg/dL) at baseline (OR, 0.133; 95% CI, 0.022–0.823; P=0.030) were inversely associated with clinical remission at week 20 (Table 2). The history of bowel resection surgery (OR, 0.123; 95% CI, 0.019–0.801; P=0.028) was the only factor significantly associated with clinical response at week 20 (Table 3).
5. AEs during UST Induction Therapy
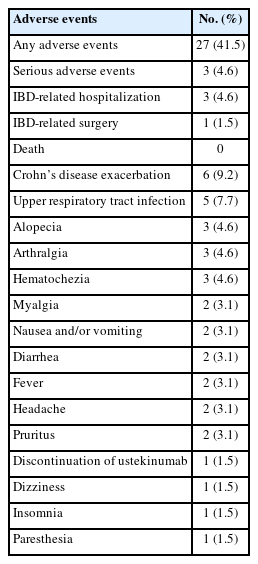
Twenty-seven of 65 patients (41.5%) experienced AEs during UST induction therapy. SAEs occurred in 3 patients (4.6%): 1 patient was hospitalized and underwent CD-related bowel surgery, and 2 patients were hospitalized due to CD exacerbation. Six patients (9.2%) experienced CD exacerbations, and one patient (1.5%) stopped UST due to a poor response. Five patients (7.7%) experienced upper respiratory tract infections. Other AEs, such as arthralgia and fever, occurred in less than 5% of patients. Details of AEs are summarized in Table 4.
DISCUSSION
In this Korean real-world, multicenter, prospective study, the clinical remission and response rates at week 20 after commencing UST induction therapy for patients with active CD were 55.1% and 71.4%, respectively. Underweight and the elevated CRP at baseline were negatively associated with clinical remission at week 20, and the history of bowel resection surgery was negatively associated with the clinical response at week 20. UST induction therapy was generally well tolerated, with only 4.6% of patients experiencing SAEs.
After placebo-controlled randomized clinical trials on the efficacy and safety of UST for patients with CD [9-11], there have been multiple real-world studies on the effectiveness and safety of UST for patients with CD, mostly conducted in the Western world [12-21]. A recent meta-analysis reported clinical remission and response rates at weeks 8 to 16 to be 34% (95% CI, 26%–42%) and 60% (95% CI, 53%–67%), respectively [36]. Most previous studies have used physicians’ assessments [12,21] or Harvy-Bradshaw index evaluations [13-19] and have evaluated clinical outcomes at weeks 8 to 16 after starting induction therapy [12-19,21].
However, because responses to UST induction therapy are scheduled for evaluations at weeks 16 to 20 according to the Korean governmental reimbursement criteria for UST therapy (after intravenous UST at week 0 and subcutaneous UST at week 8), and the first UST maintenance dosing is scheduled for week 20, we assessed the clinical remission and response rates of study patients at week 20. Additionally, because the Korean reimbursement criteria use CDAI-70 (and not Harvy-Bradshaw index) for assessing responses to UST induction therapy, we used CDAI for defining clinical outcomes. In a recent Hungarian multicenter, prospective study, patients were treated with UST using the same dose and schedule as those used in our study, and clinical remission and response rates were evaluated at weeks 16 to 20 using the same CDAI definition used by our study [20]. At week 8, clinical remission and response rates as per CDAI were 57.7% and 78.1%, respectively [20]. At weeks 16 to 20, clinical remission and response rates were 64.7% and 77.9%, respectively [20]. The Hungarian investigators observed slightly higher clinical remission and response rates than we did. This could be explained by the fact that the Hungarian study included all patients with clinical remission at baseline (14.1% of the total study cohort) and by the fact that a substantial proportion of patients were still on corticosteroids at week 8 [20]. In fact, the corticosteroid-free clinical remission rate at week 8 was 43.8%, which was lower than our corresponding finding [20]. Additionally, differences in patient characteristics, including phenotype and previous treatment, might have been associated with different outcomes.
There have been limited studies on the efficacy or effectiveness of UST induction therapy for Asian patients with CD. A Japanese subpopulation analysis of phase 3 induction and maintenance studies showed generally consistent results with those in the overall population [37]. Real-world studies on the effectiveness of UST for Asian patients with CD have previously been limited to Japanese populations [23-25]. In a retrospective, observational, single-center study of 47 patients with CD, clinical remission rates (CDAI < 150) at weeks 8 and 24 were 44.4% (8/18 patients with CDAI ≥ 150 at baseline) and 66.7% (12/18 patients with CDAI ≥ 150 at baseline), respectively [23]. In another study in which 22 patients with moderate-to-severe CD received intravenous UST therapy every 8 weeks, the rates of clinical remission and response rates were 31.8% and 59.1% at week 8 and 45.5% and 68.2% at week 24, respectively [24]. In an interim analysis of post-marketing surveillance data from Japan, among 130 CD patients with CDAI ≥ 150 at baseline, clinical remission (CDAI ≤ 150) and clinical response (decrease in CDAI ≥ 100 points) were observed in 48.5% and 40% of patients, respectively [25]. Our study provides more real-world evidence for the effectiveness of UST treatment for East Asian patients with CD.
In the present study, there was a significant decrease in CDAI during UST induction therapy. Serum albumin was significantly higher at week 8 compared with baseline, but no significant difference was noted at week 20; serum CRP was significantly lower than baseline at both week 8 and week 20; in contrast, fecal calprotectin did not show significant differences from baseline during UST induction therapy. Previous studies have yielded conflicting results regarding changes in serum CRP during UST induction therapy: decreasing CRP in several studies [12,15,17,23,38-42] and no significant change of CRP in others [14,43]. Conflicting results have also been observed for fecal calprotectin, with decreasing fecal calprotectin [15,39,41,42] or no significant change in this marker [14,43]. Further studies with more patients will be required to confirm the biochemical response during UST induction treatment.
In the present study, underweight at baseline and elevated CRP at baseline were inversely associated with clinical remission at week 20. Also, the history of bowel resection surgery was inversely associated with the clinical response at week 20. Both underweight and elevated CRP might reflect CD activity, leading to poor responses to UST [44,45]. In the case of underweight, Liefferinckx et al. [17] also found that a BMI < 18 kg/m2 was negatively associated with clinical remission at 1 year of UST therapy among CD patients with prior exposure to biologics. In contrast, Wong et al. [46] found that underweight (BMI < 18.5 kg/m2) had no impact on clinical efficacy at week 44 in a post hoc analysis of the IM-UNITI trial. These contradictory results have also been observed regarding anti-TNF therapy for CD patients [47]. In line with our results, Dulai et al. [48] reported that high baseline serum albumin, never smoking, the absence of prior exposure to anti-TNF agents, the absence of active fistulizing disease at baseline, and the absence of prior bowel surgery were predictive of clinical remission at week 16 of UST therapy. Other positive predictors of response or remission after UST induction therapy were UST therapy due to adverse effects of biologics [15,19],UST initiation due to secondary failure to biologics [19], as well as primary nonresponse to anti-TNFs [38] and concomitant immunosuppressant therapy [12]. Conversely, reported negative predictors include old age [19], smoking [19], a higher number of previous anti-TNF agents [15], and endoscopic CD severity [15].
We reported AEs and SAEs in 41.5% and 4.6% of study patients, respectively. These rates were consistent with previous observations. In the UNITI trials, AEs and SAEs were observed in 59.5% and in 5.1% of UST-treated patients, respectively [22]. In a subpopulation analysis of the UNITI trial, AEs occurred in 46.4% and SAEs in 3.6% of Japanese UST-treated patients [37]. In a recent systematic review and meta-analysis of observational studies, among UST-treated IBD patients, AEs and SAEs were observed in 16.7% (498/1,977) and 5.6% (89/1,534) of patients [36]. Although reported rates of AEs and SAEs vary by study, probably due to definitions of events and underreporting, our study adds more evidence for the safety profile of UST for Asian IBD patients.
This was the first study investigating the effectiveness and safety of UST induction therapy for Korean patients with CD. The data were prospectively collected from 4 centers using strictly predefined outcome definitions. However, there were several limitations to this study. First, the sample size was small. For this reason, the statistical power for each analysis may be insufficient, and it was particularly difficult to detect significant changes in biochemical marker levels. Second, because patients were enrolled from 4 centers, there might have been institution-specific differences in management strategies. However, because biologics treatment for patients with CD is strictly regulated by a single universal reimbursement criterion by the Health Insurance Review and Assessment Service of Korea, the general management strategy might not have varied substantially between centers. Moreover, some heterogeneity between hospitals might better reflect the real-world situation. Third, we could not present the data on endoscopic outcomes after UST induction therapy. This is because in Korea, the reimbursement criteria for evaluating UST induction therapy are based on CDAI, which does not require endoscopic evaluation. Therefore, endoscopic evaluations are rarely performed after UST induction therapy for patients with CD in the real-world practice of Korea. Finally, the follow-up duration was relatively short because we only analyzed the outcome of UST induction therapy. We plan to analyze the results of maintenance therapy with a longer follow-up duration in a subsequent study.
In conclusion, UST induction therapy was clinically effective for Korean patients with CD. The safety profile during UST induction therapy was acceptable with no new AEs. In future studies, it will be necessary to analyze the long-term effectiveness and safety of UST maintenance therapy for Asian patients with CD.
Notes
Funding Source
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2021R1A2C2095096).
Conflict of Interest
Ye BD has received a research grant from Celltrion and Pfizer Korea; consulting fees from Abbvie Korea, Celltrion, Chong Kun Dang Pharm., CJ Red BIO, Daewoong Pharma., Ferring Korea, Janssen Korea, Kangstem Biotech, Medtronic Korea, Pfizer Korea, Shire Korea, Takeda Korea, IQVIA, Cornerstones Health, and Takeda; speaking fees from Abbvie Korea, Celltrion, Ferring Korea, Janssen Korea, Pfizer Korea, Shire Korea, Takeda Korea, and IQVIA. Yang SK has received a research grant from Janssen Korea. None of the above-mentioned grants are related to this study. Other authors have nothing to declare.
Kim YS is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Data Availability Statement
Not applicable.
Author Contribution
Conceptualization: Ye BD. Project administration: Ye BD. Data collection: Oh K, Hong HS, Ham NS, Park SH, Yang SK, Yoon H, Kim YS, Choi CH, Ye BD. Analysis and interpretation of the data: Oh K, Hong HS, Lee J, Ye BD. Writing - original draft: Oh K, Hong HS. Writing - review and editing: Ye BD. Approval of the final manuscript: Oh K, Hong HS, Ham NS, Lee J, Park SH, Yang SK, Yoon H, Kim YS, Choi CH, Ye BD. Approval of final manuscript: all authors.
Non-Author Contribution
Dr. Joon Seo Lim from the Scientific Publications Team at Asan Medical Center provided editorial assistance in preparing this manuscript.