|
 |
- Search
| Intest Res > Volume 21(1); 2023 > Article |
|
Abstract
Background/Aims
Exacerbating factors of ulcerative colitis (UC) are multiple and complex with individual influence. We aimed to evaluate the efficacy of disease control by searching and restricting inflammation trigger factors of UC relapse individually in daily clinical practice.
Methods
Both patients with UC history or new diagnosis were asked to avoid dairy products at first doctor visit. Individual-reported potential trigger factors were restricted when UC flared up (Mayo endoscopy score Ōēź1) from remission status. The remission rate, duration to remission and medication were analyzed between the groups of factor restriction complete, incomplete and unknown.
Results
The total remission rate was 91.7% of 108 patients with complete restriction of dairy product. The duration to remission of UC history group was significantly longer than that of new diagnosis group (88.5 days vs. 43.4 days, P=0.006) in patients with initial endoscopic score 2-3, but no difference in patients with score 1. After first remission, the inflammation trigger factors in 161 relapse episodes of 72 patients were multiple and personal. Milk/dairy products, herb medicine/Chinese tonic food and dietary supplement were the common factors, followed by psychological issues, non-dietary factors (smoking cessation, cosmetic products) and discontinuation of medication by patients themselves. Factor unknown accounted for 14.1% of patients. The benefits of factor complete restriction included shorter duration to remission (P<0.001), less steroid and biological agent use (P=0.022) when compared to incomplete restriction or factor unknown group.
Ulcerative colitis (UC), is a chronic, complex, and common gastrointestinal inflammatory disease [1], which is characterized by a periodic relapsing and remitting course [2]. Its etiology remains unclear. The clinical goal of UC therapy is to induce and maintain remission. Any exacerbating factors related to clinical relapse should be avoided, but these factors are uncertain and difficult to control well. In recent years, the incidence and prevalence of UC have dramatically increased in Asian countries including Taiwan where the incidence was low in the past [3,4]. While genetics can only explain 7.5% of disease variance [5], environmental factors, such as diet, are believed to trigger disease onset and progression [6]. Diet not only affects the homeostasis of the gut microenvironment but also influences the gut microbial composition and function, gut barrier, and host immunity [7,8]. Especially, Western-style diets contained contain higher calories from sugar, refined carbohydrates, animal proteins, and ultra-processed foods are the major factors contributing to higher UC occurrence [9]. Thus, if we restrict these ŌĆ£specificŌĆØ diets completely, it may aid in disease control [10].
In 1961, Dr. Truelove [11] indicated that UC may be provoked by milk. As the economy grows, milk and its derivatives have become one of the most popular foods in Asia. Taiwan, following Japan, had the second-highest dairy consumption in East Asia for the past decades [12]. An epidemiological study in Japan reported that the daily intake of dairy products and meat increased, paralleling the increase in the incidence of UC [13]. Inflammatory bowel disease (IBD) patients commonly limit dairy products due to disease activity, gastrointestinal symptoms, and disease extension [14,15]. Although the dietary recommendation and restriction are popular for IBD patients [16], only a few well-designed randomized controlled trials have been done to investigate the role of diet in the management of UC [17]. Dietary factors can potentially positively or negatively affect IBD [18] and may play a role in triggering the disease [19,20]. A survey from the United Kingdom and New Zealand revealed that the majority of physicians seldom give diet advice and food exclusion suggestions to IBD patients [21]. This is probably due to the lack of high-quality evidence-based dietary guidelines [22].
The components of food are too complicated to analyze. The inflammation could be triggered by multiple factors rather than a single factor which has the influence of individual differences. The same factor may not be a one-size-fits-all pathogen. Therefore, we assume that only individual-targeted advice will be helpful in UC control. Here, we present our clinical practice by searching any changes in the patientŌĆÖs diet, tonic food, drugs, daily necessities, work and lifestyle when inflammation becomes worse during follow-up periods. We aim to find the possible inflammation trigger factors of UC relapse, analyze the treatment outcome by restricting these factors individually.
This was a retrospective study to evaluate the effectiveness of UC control with dairy products restriction first and possible inflammation trigger factors related to UC relapse. We analyzed the treatment effect by restriction of trigger factors through the chart review. These data were collected according to the chart records of UC patients treated in the outpatient clinic at Chang Gung Memorial Hospital, Linkou, Taiwan, by a single colorectal specialist from January 2006 and December 2019. The studyŌĆÖs protocol was approved by the Chang Gung Medical Foundation Institutional Review Board (IRB No. 202100587B0). The informed consent was waived. The initial UC diagnosis was based on endoscopic and pathologic findings with chronic and acute inflammation of mucosa and submucosa layer. The blood tests including complete blood count, white blood cell differential count, erythrocyte sedimentation rate, C-reactive protein and amebiasis titer were performed to exclude possible infective colitis. The patients with CrohnŌĆÖs disease (CD), nonspecific colitis or diagnosed as UC with a follow-up duration of less than 6 months were excluded.
Inflammation severity of UC was assessed based on Mayo endoscopic score system. In this study, we defined an endoscopic score of < 1 (normal mucosa pattern to mild hyperemic mucosa patch without light touch bleeding) as remission status, and an endoscopic score of 1-3 (mild, moderate, and severe) with rectal bleeding as the active disease. A relapse episode meant that the patientŌĆÖs Mayo endoscopic score increased from < 1 to 1-3 and then returned to < 1 after the intervention. The intervention included specific diet restrictions, medication, and surgery.
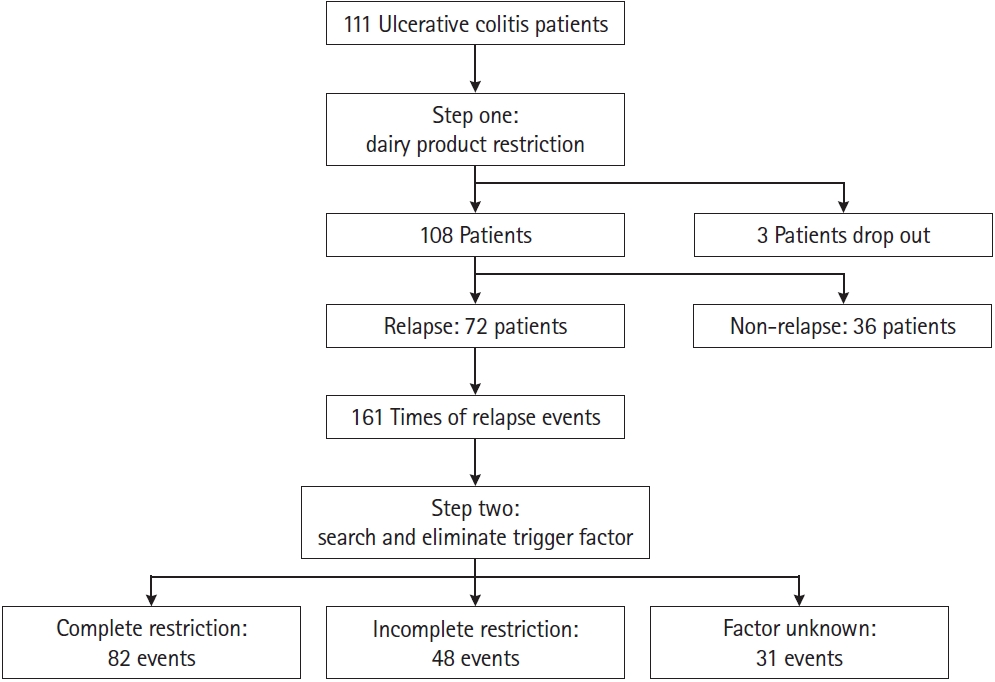
Our protocol was designed as follows (Fig. 1).
At first, we started our clinical observation with the avoidance of dairy products (milk, bread, cake, cookie, cheese, cream, ice cream, etc.). We had asked the patients with UC history and found no advisement to restrict dairy products from doctors before in other hospitals. All patients, including both newly diagnosed and those with a history of UC, were asked to avoid the dairy diet at their first doctor visit. Patients were then followed up weekly or monthly to monitor disease severity by rigid sigmoidoscopy at the outpatient clinic for the next 6 months.
Rigid sigmoidoscopy also was performed at regular followup visits with an interval of 3 to 6 months. The colonoscopy examination was arranged with an interval of 1 to 5 years or at the time of inflammation deterioration. Disease severity was examined by sigmoidoscopy or colonoscopy, completion of diet restriction, duration to remission, and medication use were recorded.
Next, all patients were regularly followed up. Once the disease relapse was confirmed by colonoscopy or sigmoidoscopy evaluation, we interviewed each patient in detail to search for any changes in the patientŌĆÖs diet, tonic food, drug, daily necessities, work, and lifestyle before the time of relapse to find possible inflammation trigger factors. When restricting these factors can decrease the inflammation severity back to remission status, these factors were recorded as inflammation trigger factors. Physicians then requested patients to eliminate these possible factors to observe whether UC inflammation could subside faster. Disease severity, completion of factor restriction, duration of remission, and medication use were reviewed. Multiple factors were defined as 2 or more factors reported in a single relapse episode.
According to patientŌĆÖs compliance, we classified patients into factor complete restriction, incomplete restriction and unknown 3 groups to evaluate the effect of inflammation control. Complete restriction means totally no contact with the trigger factors. Incomplete restriction means that patient will not totally obey the advisement, only decrease the frequency of factor contact, or need several times outpatient department follow-up and advisement to achieve totally restriction. Unknown means we cannot find trigger factors. During follow-up periods, we upgraded the drug use by increasing the mesalazine dosage, adding steroids, azathioprine or biological agents to control the inflammation if UC relapse. If the inflammation degree of patients returned to only mild hyperemic mucosa patches without rectal bleeding or present vascular pattern, we downgraded the drug use by decreasing the mesalazine dosage to less than 2 g per day, tapering the steroid, azathioprine or biological agent. Almost all the proctitis patients with mild inflammation were used mesalazine suppository to control the disease.
We used SPSS statistics software (version 24.0; IBM Corp., Armonk, NY, USA) for statistical analysis. A comparative analysis between the groups was performed using an independent samples t-test for continuous variables and a chi-square test, or Fisher exact test for categorical variables. Statistical significance was set at P< 0.05.
A total of 108 patients (73 men, mean age 45.8 ┬▒ 13.6 years) were enrolled in this study. The follow-up duration ranged from 228 days to 13 years with a mean duration of 6.2 ┬▒ 3.8 years. The patients were categorized into 2 groups, new diagnosis (n = 59, 53.2%) and UC history (n = 52, 46.8%). The periods from UC diagnosis to the beginning of the study was ranged from 0.22 to 15.5 years with a mean time of 5.44 ┬▒ 3.76 years in the UC history group. Patient characteristics are shown in Table 1. There were no significant differences in age, sex, and Mayo endoscopic score at the first clinic visit between the 2 groups. The percentage of proctitis was significantly higher in the new diagnosis group, and pancolitis was more in the UC history group. The patients with steroid use before the first doctor visit was more in UC history group compared to new diagnosis group (28 vs. 1, P< 0.001). The only patient who used steroids in the new diagnosis group was due to the treatment of rheumatic arthritis. Most patients in new diagnosis group only needed mesalazine and dairy restriction for disease control.
Table 2 showed the treatment outcome after dairy product restriction plus medication. The total remission rate was 91.7% (99/108 patients). The remission rates of UC history and new diagnosis groups were 91.3% and 93.9% in patients with the initial endoscopic score 1 (P= 0.706), and 75.8% and 96.1% in patients with scores 2-3 (P= 0.033), respectively.
The mean duration of achieving remission was approximately 40 days in both group patients with endoscopic score 1 (P= 0.387), but was 88.5 ┬▒ 68.9 days of UC history group with scores 2-3 which was significantly twice longer than 43.4 ┬▒ 33.5 days of the new diagnosis group (P= 0.006). Some patients with initial endoscopic score 1 could be medication-free after remission. The medication-free rate of the new diagnosis group was 30.3% (10/33), higher than 13.0% (3/23) of UC history group but without a statistical significance (P= 0.132). The use of steroid decreased with an overall tapering rate of 46.7% (7/15) in patients with UC history group. However, all the patients with new diagnosis could be steroid-free (P= 0.015).
At the end of the study, 36 patients with a follow-up duration of 6.24 ┬▒ 3.82 years never experienced flare-ups again. All of them restricted dairy products very well. Although some patients still needed to take low-dose mesalazine ( Ōēż 2 g) per day, 36% (13/36) of them were medication-free.
Seventy-two patients had experienced at least 1 to 7 relapse episodes individually with a total of 161 episodes during follow-up period with a mean of 6.20 ┬▒ 3.81 years. After relapse, we searched the possible inflammation trigger factors and restrict them to aid disease control (Table 3). When relapse happened, the percentage of steroid use were 45.8% and 48.4% of the factor incomplete restriction and unknown groups respectively which was significantly higher than 39.0% of the factor complete restriction group (P= 0.022). The steroid tapering rates were 59%, 41%, and 60% in the complete restriction, incomplete restriction, and factor unknown groups, respectively. There was no difference in steroid tapering rate between the 3 groups. The patients who needed biological agent (adalimumab) to control inflammation after relapse were still difficult to spare it for disease control even achieved remission status. The mean duration to remission of the factor complete restriction group was 46.8 ┬▒ 42.1 days which was significant shorter than 209.0 ┬▒ 164.9 and 98.0 ┬▒ 102.5 days of the factor incomplete restriction and unknown groups (P< 0.001). Surgical intervention was performed in 2 patients (1 in factor incomplete restriction and 1 in factor unknown group) because of acute abdomen events after several episodes of relapse.
Among the 72 patients with relapse, 28 patients experienced only 1 relapse and the others had 2 to 7 episodes during follow-up. We found that the possible trigger factors were highly variable (Table 4). Milk, dairy product, herb medicine/Chinese tonic food, and dietary supplement were the more common trigger factors. But there were still 14.1% of patients who cannot find trigger factors. Some patients would be exposed to the same risk factor that had been self-reported after the disease inflammation subsided. Milk and dairy products (a total of 13 repeats) were reported as the most common repeated factors in the same patient, followed by herbal medicine and Chinese tonic food (6 repeats).
The inflammation trigger factors related to UC relapse are multiple and complex with individual and personal variance. The possible biological mechanisms of environmental and psychological factors on clinical relapse of IBD included the increase of thrombotic tendency, imbalances in prostaglandin synthesis, alternations in the intestinal microbiome, mucosal damage with mucus layer disruption and increase of intestinal permeability [23,24]. Restriction of some diet and fecal implantation were associated with a decrease of UC relapse. In contrast, cease of smoking, nonsteroidal anti-inflammatory drugs (NSAIDs), dietary sulfur, high ratio of n-6/n-3 polyunsaturated fatty acids, deficiency of vitamin D and high-stress level increased the possibility of UC relapse. Estrogens, antibiotics and air pollution were supposed but still lacked supportive evidence.
Both doctors and patients may perceive that diet is one of the important environmental triggers of UC relapse. An India survey reported that patients perceived food as a risk factor (44%), and modified their diet since the diagnosis (86%). The diet modification included taking vitamin, herbal medication, mineral, nutritional and fiber supplements. The food restrictions were imposed by 89.9% of patients for the belief of preventing relapse. While more CD patients avoided raw fruits (P= 0.002), more UC patients avoided dairy products (P= 0.013) and took more fiber supplements (P= 0.049) [25]. Our patients with similar Asia cultures also would seek dietary supplements, Chinese tonic food, and herbal medicine to control the disease by themselves without any cognition of getting better or worse. The behavior of patients to modify their diet is very diverse. It is difficult to control 1 dietary factor without the influence of others. Although the benefits of dietary therapy by exclusive enteral nutrition was proved in CD [8], the benefits in UC was uncertain [26].
Milk contains an abundance of casein, which is known to negatively affect intestinal permeability and gut microbial density in mice [27]. Dietary restriction of milk and its derivatives is recommended [18]. In 1965, Wright and Truelove [28] first conducted a 1-year randomized clinical trial and reported that a milk-free diet could not significantly decrease the relapse episodes. However, some symptom-free patients with a milk-free diet had a rapid relapse after reintroduction of milk into the diet. These patients responded well to a short course of corticosteroids plus a milk-free diet. Although subsequent clinical researches cannot prove the association of milk and milk products with UC relapse [29,30], the contents of dairy foods such as margarine [31], emulsifiers [32,33], and carrageenan [34] were related to UC inflammation and a milk-free diet was beneficial to a certain proportion of UC patients. Our findings revealed dairy products were the most frequent factors related to UC relapse and their restriction could shorten the treatment course to achieve remission faster.
Chinese herbal medicine and herbal tonic foods are widely used for diseases and body strengthening among a large proportion of Asian people. The use of herbal therapy for IBD is growing worldwide [35]. Certain types of herbal medicines as alternative therapy could be effective in UC induction and maintenance of remission [36]. However, herbal medicine and tonic food were the second most common trigger factors reported from our patients. Because the variety of products lack manufacturing standards and clinical research support.
Dietary supplements and probiotics are commonly used for health benefits by patients. Low serum vitamin D during remission increased the risk of clinical relapse in UC patients [37]. But the effect of vitamin D supplement therapy still is not clear. Curcumin, an Asian spice with antioxidant and anti-inflammatory properties, in combination with mesalamine was superior to mesalamine alone to induce remission in patients with mild to moderate UC from a randomized trial [38]. Probiotic bacteria play a crucial role in maintaining gut microbiota homeostasis, strengthening gut barrier function and host immune responses. An updated review summarized the probiotics used alone or in combination with conventional therapy against UC [39]. These probiotics included the Escherichia coli Nissle 1917, Lactobacillus rhamnosus GG (LGG), Bifidobacterium longum 536, and the multi-strain BIFICO, BIO-THREE and VSL#3. However, the Cochrane Database review reported the uncertain effectiveness of probiotics for maintaining UC remission due to low- to very low- certainty evidence from poorly conducted studies [40]. In our study, some dietary supplements including probiotics taken by patients themselves were the trigger factors of UC relapse. Physicians should be aware that these unidentified products with unknown efficacy may aggravate the symptoms of patients.
Alcohol, processed meat, and other varied products also were the reported factors in our study. Alcohol was associated with UC disease activity and the sulfite used in processed alcoholic drinks was believed to be a key factor [29,41]. The ultra-processed foods [42,43] and others with undetermined components may interfere intestinal permeability or microbiome and worsen clinical symptoms. For example, carrageenan, an ingredient used in preparing dairy products, dessert, meat, beer, and even toothpaste and shampoo, was a contributory cause of UC relapse faster [34].
The psychological problems such as anxiety [44], depression [45], fatigue [46], insomnia [47], and stress [48] are often noted in IBD patients, especially when the disease flares up. The perception of stress, negative mood, and major life events were the trigger factors for IBD flares [49]. Psychotherapeutic intervention is helpful for the quality of life but still is uncertain for disease control.
We observed that some UC patients with mild inflammation can decrease or withdraw mesalazine usage after restricting trigger factors. But the discontinuation of the medication by patients themselves (mesalazine) is another trigger factor of UC relapse. Although the potential effect of 5-aminosalicylates maintenance therapy can reduce cancer risk, withdrawal or intermittent use of 5-aminosalicylates is possible in special subgroup patients after long-term remission status [50]. Cigarette smoking seems to be a protective factor against UC [31], and smoking cessation may act as a triggering factor [51,52]. Oral contraceptive pills and postmenopausal hormone therapy were associated with IBD development in women [53]. The risk increase of UC depended on the duration of hormone use and risk dropped due to discontinuation of hormone therapy (estrogen or estrogen plus progestin) [54]. Our findings revealed that the disease progression was related to the use of high fluoride toothpaste or mouthwash, cosmetic agents, hair dye, and hairspray which were not reported before. The mechanism was unknown but the inflammation gradually subsided after the discontinuation of these products.
We cannot find out possible trigger factors for 14.1% of patients with UC relapse. Further histologic or serologic examination including immune and infective conditions will be necessary to evaluate the etiology of disease progression.
In conclusion, The exacerbating factors of UC are multiple and complex with personal variance and influence of individual factors. We cannot inform the patients to restrict all the possible factors completely and expect the efficacy by controlling a single factor. In daily clinical practice, restriction of dairy diet, undefined alternative therapies, chemical and hormonecontent products first then searching and restriction trigger factors personally if UC relapse can improve the disease control and downgrade the medication usage of UC patients.
This study had some limitations. Except for the restriction of dairy diet initially, the data of inflammation trigger factors obtained from the questions to patients and answers when UC relapse. We cannot quantify these factors and evaluate the influence of each factor individually. The trigger factors recorded were according to the efficacy of factor restriction. The patientŌĆÖs compliance with factor restriction was not the same and the classification of complete restriction was dependent on the patientŌĆÖs answer at the first follow-up visit after UC relapse. NSAIDs are the potential trigger factors of UC relapse. The regular use of aspirin cannot be discontinued and short-term use of NSAIDs was not recorded clearly. The priority of drug upgraded to treat the UC relapse was a steroid. Rare patients used immunomodulators or biological agents in this study.
ADDITIONAL INFORMATION
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Author Contribution
Conceptualization: Tsai WS. Methodology: Tsai WS. Formal analysis: Tsai KY, Huang SH. Project administration: Tsai WS. Supervision: You JF, Chern YJ, Hsu YJ, Tsai WS. Visualization: Tsai TY. Writing-original draft: Tsai KY. Writing-review and editing: Tsai WS. Approval of final manuscript: all authors.
Table┬Ā1.
Clinical Characteristics in Patients with History and New Diagnosed of Ulcerative Colitis
| Characteristics | History of ulcerative colitis (n=52) | New diagnosis (n = 59) | P-value |
|---|---|---|---|
| Age at diagnosis (yr) | 40.6 ┬▒ 15.5 | 45.1 ┬▒ 12.4 | 0.188 |
| Sex | 0.128 | ||
| ŌĆāMale | 38 | 35 | |
| ŌĆāFemale | 14 | 24 | |
| Disease extension | 0.033 | ||
| ŌĆāProctitis | 19 | 35 | |
| ŌĆāLeft colitis | 22 | 19 | |
| ŌĆāPancolitis | 11 | 5 | |
| Initial Mayo endoscopic score | 0.236 | ||
| ŌĆāMayo score 1 | 23 | 33 | |
| ŌĆāMayo score 2 | 18 | 21 | |
| ŌĆāMayo score 3 | 11 | 5 | |
| Steroid use before the first outpatient department visit | 28 | 1a | < 0.001 |
| Medication prescribed at the first visit with diary product restriction | < 0.001 | ||
| ŌĆāMesalazine only | 37 | 57 | |
| ŌĆāMesalazine and steroid | 15 | 2 |
Table┬Ā2.
The First Remission Information after Dairy Product Restriction
| Variable | History of ulcerative colitis | New diagnosis | P-value |
|---|---|---|---|
| Initial endoscopic score 1 | |||
| ŌĆāNo. of participants | 23 | 33 | |
| ŌĆāRemission achieved | 21 (91.3) | 31 (93.9) | 0.706 |
| ŌĆāDuration to remission (day) | 40.4 ┬▒ 26.2 | 39.9 ┬▒ 32.5 | 0.387 |
| ŌĆāMedication at remission | 0.256 | ||
| ŌĆāŌĆāMesalazine only | 16 | 20 | |
| ŌĆāŌĆāMesalazine and steroid | 2 | 1a | |
| ŌĆāŌĆāNo medication | 3 | 10 | 0.132 |
| Initial endoscopic scores 2-3 | |||
| ŌĆāNo. of participants | 29 | 26 | |
| ŌĆāRemission achieved | 22 (75.8) | 25 (96.1) | 0.033 |
| ŌĆāDuration to remission (day) | 88.5 ┬▒ 68.9 | 43.4 ┬▒ 33.5 | 0.006 |
| ŌĆāMedication at remission | 0.015 | ||
| ŌĆāŌĆāMesalazine only | 16 | 24 | |
| ŌĆāŌĆāMesalazine and steroid | 6 | 0 | |
| ŌĆāŌĆāNo medication | 0 | 1 |
Table┬Ā3.
The Relapse and Remission Episode with Possible Factor Control
| Management when relapse | Factor complete restriction (n = 82) | Factor incomplete restriction (n = 48) | Factor unknown (n = 31) | P-value |
|---|---|---|---|---|
| Mesalazine | 50 (60.9) | 24 (50.0) | 12 (38.7) | 0.022 |
| Mesalazine and steroid | 32 (39.0) | 22 (45.8) | 15 (48.4) | |
| Mesalazine and adalimumab | 0 | 1 (2.1) | 3 (9.7) | |
| Duration to remission (day) | 46.8 ┬▒ 42.1 | 209.0 ┬▒ 164.9 | 98.0 ┬▒ 102.5 | < 0.001 |
| Steroid tapera | 19 (59.3) | 9 (40.9) | 9 (60.0) | 0.350 |
| Adalimumab taperb | NA | 0 | 0 | NA |
| Surgical intervention | 0 | 1 (2.1) | 1 (0.3) | 0.316 |
Table┬Ā4.
Possible Trigger Factors of Ulcerative Colitis Flare Up
REFERENCES
2. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017;11:649-670.



3. Kuo CJ, Yu KH, See LC, et al. The trend of inflammatory bowel diseases in Taiwan: a population-based study. Dig Dis Sci 2015;60:2454-2462.



4. Wei SC, Chang TA, Chao TH, et al. Management of ulcerative colitis in Taiwan: consensus guideline of the Taiwan Society of Inflammatory Bowel Disease. Intest Res 2017;15:266-284.




5. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet 2017;389:1756-1770.


6. Geerling BJ, Dagnelie PC, Badart-Smook A, Russel MG, Stockbr├╝gger RW, Brummer RJ. Diet as a risk factor for the development of ulcerative colitis. Am J Gastroenterol 2000;95:1008-1013.


7. Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol 2011;106:563-573.



8. Levine A, Sigall Boneh R, Wine E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018;67:1726-1738.


9. Chiba M, Nakane K, Komatsu M. Westernized diet is the most ubiquitous environmental factor in inflammatory bowel disease. Perm J 2019;23:18-107.



10. Witkowski M, Witkowski M, Gagliani N, Huber S. Recipe for IBD: can we use food to control inflammatory bowel disease? Semin Immunopathol 2018;40:145-156.




12. Lee MS, Wahlqvist ML, Peng CJ. Dairy foods and health in Asians: Taiwanese considerations. Asia Pac J Clin Nutr 2015;24 Suppl 1:S14-S20.

13. Kitahora T, Utsunomiya T, Yokota A. Epidemiological study of ulcerative colitis in Japan: incidence and familial occurrence. The Epidemiology Group of the Research Committee of Inflammatory Bowel Disease in Japan. J Gastroenterol 1995;30 Suppl 8:5-8.

14. Brasil Lopes M, Rocha R, Castro Lyra A, et al. Restriction of dairy products: a reality in inflammatory bowel disease patients. Nutr Hosp 2014;29:575-581.

15. Limdi JK. Dietary practices and inflammatory bowel disease. Indian J Gastroenterol 2018;37:284-292.




16. Hou JK, Lee D, Lewis J. Diet and inflammatory bowel disease: review of patient-targeted recommendations. Clin Gastroenterol Hepatol 2014;12:1592-1600.



17. Keshteli AH, Madsen KL, Dieleman LA. Diet in the pathogenesis and management of ulcerative colitis: a review of randomized controlled dietary interventions. Nutrients 2019;11:1498.



18. Rizzello F, Spisni E, Giovanardi E, et al. Implications of the westernized diet in the onset and progression of IBD. Nutrients 2019;11:1033.



19. Limdi JK, Aggarwal D, McLaughlin JT. Dietary practices and beliefs in patients with inflammatory bowel disease. Inflamm Bowel Dis 2016;22:164-170.


20. Yoon JY. Nutritional approach as therapeutic manipulation in inflammatory bowel disease. Intest Res 2019;17:463-475.




21. Inns SJ, Emmanuel AV. Survey of UK and New Zealand gastroenterologistsŌĆÖ practice regarding dietary advice and food exclusion in irritable bowel syndrome and inflammatory bowel disease. Frontline Gastroenterol 2013;4:44-50.



22. Limketkai BN, Iheozor-Ejiofor Z, Gjuladin-Hellon T, et al. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst Rev 2019;2:CD012839.



23. Martin TD, Chan SS, Hart AR. Environmental factors in the relapse and recurrence of inflammatory bowel disease: a review of the literature. Dig Dis Sci 2015;60:1396-1405.



24. Vedamurthy A, Ananthakrishnan AN. Influence of environmental factors in the development and outcomes of inflammatory bowel disease. Gastroenterol Hepatol (NY) 2019;15:72-82.
25. Tomar SK, Kedia S, Upadhyay AD, et al. Impact of dietary beliefs and practices on patients with inflammatory bowel disease: an observational study from India. JGH Open 2017;1:15-21.




26. Gkikas K, Gerasimidis K, Milling S, Ijaz UZ, Hansen R, Russell RK. Dietary strategies for maintenance of clinical remission in inflammatory bowel diseases: are we there yet? Nutrients 2020;12:2018.



27. Llewellyn SR, Britton GJ, Contijoch EJ, et al. Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology 2018;154:1037-1046.



28. Wright R, Truelove SC. A controlled therapeutic trial of various diets in ulcerative colitis. Br Med J 1965;2:138-141.



29. Jowett SL, Seal CJ, Pearce MS, et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut 2004;53:1479-1484.



30. Strisciuglio C, Giannetti E, Martinelli M, Sciorio E, Staiano A, Miele E. Does cowŌĆÖs milk protein elimination diet have a role on induction and maintenance of remission in children with ulcerative colitis? Acta Paediatr 2013;102:e273-e278.


31. Kurata JH. Dietary and other risk factors of ulcerative colitis: a case-control study in Japan. Epidemiology Group of the Research Committee of Inflammatory Bowel Disease in Japan. J Clin Gastroenterol 1994;19:166-171.

32. Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015;519:92-96.




33. Lecomte M, Couëdelo L, Meugnier E, et al. Dietary emulsifiers from milk and soybean differently impact adiposity and inflammation in association with modulation of colonic goblet cells in high-fat fed mice. Mol Nutr Food Res 2016;60:609-620.



34. Bhattacharyya S, Shumard T, Xie H, et al. A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity. Nutr Healthy Aging 2017;4:181-192.



35. Triantafyllidi A, Xanthos T, Papalois A, Triantafillidis JK. Herbal and plant therapy in patients with inflammatory bowel disease. Ann Gastroenterol 2015;28:210-220.


36. Torres J, Ellul P, Langhorst J, et al. European CrohnŌĆÖs and Colitis Organisation topical review on complementary medicine and psychotherapy in inflammatory bowel disease. J Crohns Colitis 2019;13:673-685e.



37. Gubatan J, Mitsuhashi S, Zenlea T, Rosenberg L, Robson S, Moss AC. Low serum vitamin D during remission increases risk of clinical relapse in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2017;15:240-246.



38. Lang A, Salomon N, Wu JC, et al. Curcumin in combination with mesalamine induces remission in patients with mild-tomoderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol 2015;13:1444-1449.


39. Dhillon P, Singh K. Therapeutic applications of probiotics in ulcerative colitis: an updated review. Pharma Nutrition 2020;13:100194.

40. Iheozor-Ejiofor Z, Kaur L, Gordon M, Baines PA, Sinopoulou V, Akobeng AK. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 2020;3:CD007443.



41. Magee EA, Edmond LM, Tasker SM, Kong SC, Curno R, Cummings JH. Associations between diet and disease activity in ulcerative colitis patients using a novel method of data analysis. Nutr J 2005;4:7.




43. Monteiro CA, Cannon G, Levy RB, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr 2019;22:936-941.



44. Mikocka-Walus A, Knowles SR, Keefer L, Graff L. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis 2016;22:752-762.

45. Neuendorf R, Harding A, Stello N, Hanes D, Wahbeh H. Depression and anxiety in patients with Inflammatory Bowel Disease: a systematic review. J Psychosom Res 2016;87:70-80.


46. Vogelaar L, vanŌĆÖt Spijker A, Timman R, et al. Fatigue management in patients with IBD: a randomised controlled trial. Gut 2014;63:911-918.


47. Ali T, Madhoun MF, Orr WC, Rubin DT. Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease patients. Inflamm Bowel Dis 2013;19:2440-2443.


48. Bernstein MT, Targownik LE, Sexton KA, Graff LA, Miller N, Walker JR. Assessing the relationship between sources of stress and symptom changes among persons with IBD over time: a prospective study. Can J Gastroenterol Hepatol 2016;2016:1681507.




49. Bernstein CN, Singh S, Graff LA, Walker JR, Miller N, Cheang M. A prospective population-based study of triggers of symptomatic flares in IBD. Am J Gastroenterol 2010;105:1994-2002.



50. Annah├Īzi A, Moln├Īr T. Optimal endpoint of therapy in IBD: an update on factors determining a successful drug withdrawal. Gastroenterol Res Pract 2015;2015:832395.


51. Beaugerie L, Massot N, Carbonnel F, Cattan S, Gendre JP, Cosnes J. Impact of cessation of smoking on the course of ulcerative colitis. Am J Gastroenterol 2001;96:2113-2116.


52. Jiang L, Xia B, Li J, et al. Risk factors for ulcerative colitis in a Chinese population: an age-matched and sex-matched casecontrol study. J Clin Gastroenterol 2007;41:280-284.