Ustekinumab for anti-tumor necrosis factor refractory pediatric ulcerative colitis: a promising approach towards endoscopic healing
Article information
Abstract
Background/Aims
To describe the role of ustekinumab in inducing remission and endoscopic healing in anti-tumor necrosis factor α nonresponsive pediatric ulcerative colitis patients at a tertiary care inflammatory bowel disease center.
Methods
A retrospective chart review was performed on patients with ulcerative colitis receiving ustekinumab. Primary outcome was steroidfree clinical remission at follow-up. Secondary outcomes were biochemical remission and endoscopic healing.
Results
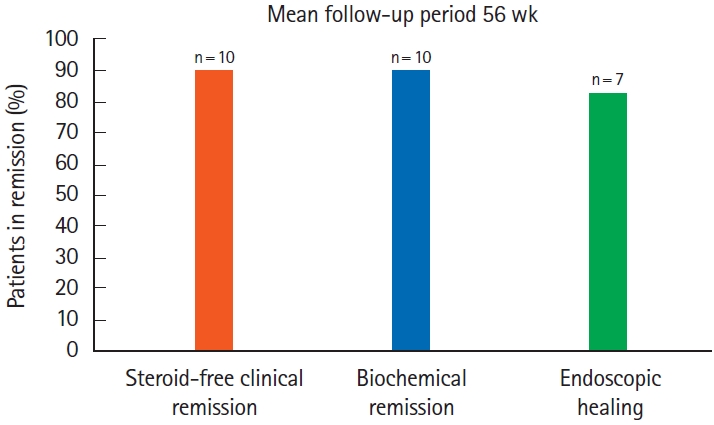
Ten children were analyzed; 7 (70%) had ulcerative colitis, and 3 (30%) had inflammatory bowel disease unspecified with colitis. Median follow-up period was 56 weeks. Nine patients (90%) achieved steroid-free clinical remission and biochemical remission. Seven patients had follow-up colonoscopies, out of which 6 (86%) achieved endoscopic remission, while 1 (14%) underwent colectomy. Out of the 3 patients without a follow-up colonoscopy, fecal calprotectin levels downtrended to < 150 mg/kg in 2 patients and < 400 mg/kg in 1 patient from baseline level of > 2,000 mg/kg.
Conclusions
Ustekinumab appears efficacious in achieving not only clinical and biochemical remission but also has promising role in inducing endoscopic healing end point in patients who fail other biologics.
INTRODUCTION
Ulcerative colitis (UC) is a chronic inflammatory condition involving the large intestine. In contrast to adults, children with UC are more likely to have widespread and severe disease, and treatment strategies beyond first-line agents (steroids and mesalamine) are often needed in children [1,2]. Anti-tumor necrosis factor α (anti-TNF-α) agents have been used in cases of moderate-to-severe UC that have failed standard treatments. However, primary non-response or secondary loss of response to anti-TNF therapy is often encountered [3-5]. Ustekinumab is a humanized monoclonal antibody targeted against the p40 subunit of interleukin (IL)-12 and IL-23 inflammatory cytokines which are involved in intestinal inflammation. This inhibits the signal transduction cascade downstream of Th17 and Th1 inflammatory pathways and thus plays an important role in immune regulation and disease control [6,7]. In 2019 ustekinumab was first approved for treatment of UC in adults after the UNIFI trial demonstrated its efficacy and safety, especially in patients with colitis refractory to anti-TNF treatment [8]. Recent studies involving large pediatric UC cohorts have shown encouraging results [9,10]. However, more longitudinal studies are needed to determine its role in sustained endoscopic healing. This retrospective study aims to evaluate the efficacy of ustekinumab in anti-TNF refractory pediatric patients with moderate-to-severe UC.
METHODS
We conducted a retrospective chart review of pediatric patients diagnosed with UC at Hasbro Children’s Hospital. We identified 10 patients who had previously failed anti-TNF agents and were below the age of 20 years at the time of induction with ustekinumab. Electronic medical records were reviewed. Baseline data including demographic variables were obtained as outlined in Table 1.

Baseline Demographic and Disease Characteristics of Our Patients at the Time of Induction with Ustekinumab
Disease phenotype was categorized according to the Paris classification, and the endoscopic severity was based on Mayo scoring during colonoscopy. Disease activity was assessed using the Pediatric Ulcerative Colitis Activity Index (PUCAI) [11]. List of prior pharmacologic treatments, use of any inflammatory bowel disease (IBD) specific dietary therapy in addition to pharmacotherapy (specific carbohydrate diet/Mediterranean diet) as well as laboratory parameters including C-reactive protein (CRP) and fecal calprotectin (FCP) at baseline and follow-up visits were obtained from the chart review.
Primary outcomes in our study included steroid-free clinical remission (SFCR) defined as PUCAI score < 10 with no concomitant steroid use > 4 weeks at the end of mean follow-up period of 56 weeks.
Secondary outcomes included improvement in inflammatory markers including CRP and FCP. Biochemical remission was defined as CRP < 10 mg/L. Endoscopic healing was defined as Mayo endoscopic score of ≤ 1.
This study was approved by the Institutional Review Board of Rhode Island Hospital (IRB ID# 1804917-3). Informed consent was waived. Data was collected and managed using the REDCap (Research Electronic Data Capture) tool hosted at Lifespan’s Department of Information Services. Univariate distribution of all variables was described. Continuous variables were reported with means and standard deviations while categorical variables were reported as frequencies and percentages.
RESULTS
During our inclusion period, 10 pediatric patients received ustekinumab treatment for colitis. Median age at diagnosis was 13.0 years (interquartile range [IQR], 11.5–16.5 years). Out of these, 7 (70%) had UC, and 3 (30%) had IBD-unclassified with colitis. All patients had failed anti-TNF agents while 2 patients (20%) also failed vedolizumab (anti-integrin) therapy in addition to anti-TNF (Table 1). All patients had active disease at baseline. The median PUCAI score was 62.5 (IQR, 55.0–70.0), median CRP was 25.9 mg/L (IQR, 9.7–55.9 mg/L) and median FCP was 2,089 mg/kg (IQR, 1,445–3,000 mg/kg) respectively at baseline. Colonoscopy was obtained in 9 patients prior to therapy escalation to ustekinumab and showed pancolitis in 8 and left-sided colitis in 1 patient. Median age at ustekinumab induction was 14.3 years (IQR, 14.0–17.5 years) with median disease duration of 1.8 years (IQR 1.5–2.0 years).
All 10 patients received a single weight-based intravenous induction dose of ustekinumab ( ≤ 55 kg: 260 mg, 56 kg–85 kg: 390 mg, and > 85 kg: 520 mg) followed by standard subcutaneous 90 mg dose at 8 weeks interval. Dose escalation was needed for 7 patients (70%) by weeks 16–20 to achieve clinical remission. Median ustekinumab trough concentration was 6.5 μg/mL (IQR, 3.5–8.9 μg/mL) obtained during the maintenance phase. Median follow-up period was 56 weeks in our study and the primary endpoint of SFCR was achieved in 9 patients (90%) (Fig. 1). Given this was a retrospective study with a small sample size of only 10 patients, we focused on providing a descriptive analysis and utilized a mixed model/growth curve for assessing PUCAI scores and other parameters. All parameters including PUCAI scores, CRP, and FCP overall downtrended after starting ustekinumab (Figs. 2, 3). We did not include erythrocyte sedimentation rate in our final analysis as it is a nonspecific marker of inflammation and can be elevated under any stress state.

(A) Individual patient C-reactive protein levels by visit (mg/L). (B) Individual patient fecal calprotectin levels by visit (mg/kg). ID refers to patient identification number. Dx, diagnosis; Ind, induction.
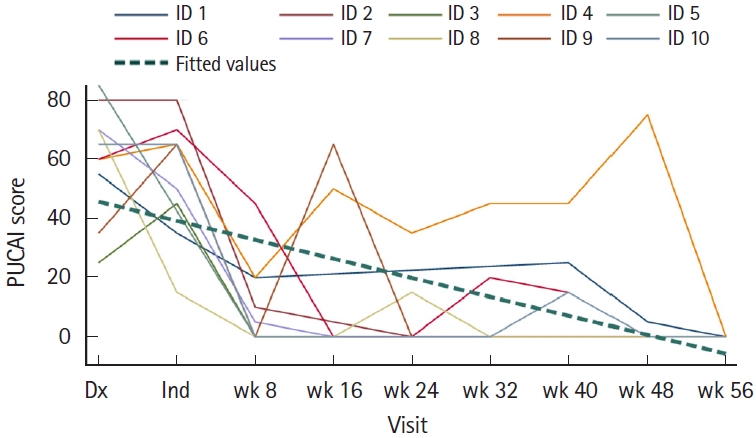
Individual patient Pediatric Ulcerative Colitis Activity Index (PUCAI) scores by visit. ID refers to patient identification number. Dx, diagnosis; Ind, induction.
Follow-up endoscopy was obtained in 7 patients and showed endoscopic healing in 6 (86%), while 1 (14%) patient (patient ID #4) had pancolitis who then underwent colectomy. This patient had also failed vedolizumab and continued to have high inflammatory markers consistent with severe disease. Out of the 3 patients without a follow-up colonoscopy, calprotectin levels downtrended to < 150 mg/kg in 2 patients indicating mucosal healing, and < 400 mg/kg in 1 patient from baseline level of > 2,000 mg/kg. None of the patients developed serious drug-associated adverse events during the follow-up period.
DISCUSSION
In our retrospective study, ustekinumab successfully induced remission in moderate-to-severe pediatric UC patients’ refractory to treatment with anti-TNF agents. SFCR was achieved by 90% of patients. This is higher than that reported in other large pediatric studies where SFCR was achieved by 44% of patients at week 52, and 62% of patients at last follow-up visit [9,10]. Possible explanations include different regimen of prescribing and tapering steroids by the providers or difference in the disease phenotype. In all these studies, failure of prior biologic therapy was associated with decreased likelihood of achieving remission [8-10]. We did not have any biological naïve group for comparison, so this could not be fully assessed. However, prior biological failure did not seem to affect the primary outcome of SFCR in our study.
The standard ustekinumab regimen includes a single weightbased intravenous dose followed by subcutaneous injection every 8 weeks. In our patient group, 70% of patients received dose escalation with increased frequency of maintenance injections to either every 4 weeks or every 6 weeks to achieve clinical remission. Our results were similar to another large pediatric study in Crohn’s disease patients where the investigators also observed the need for elevated ustekinumab trough concentrations to attain remission. Specifically, the median ustekinumab trough level, measured before any dose adjustments, mirrored our own findings, with a median of 6.2 μg/mL (IQR, 3.9–7.7 μg/mL) [12]. Among the cohort of patients in our study, those children who necessitated dose escalation exhibited pancolitis and a more severe disease profile compared to the patients who did not require such adjustments. In the past, providers have observed a similar pattern in the response of IBD patients to anti-TNF agents, where some patients may require higher drug levels despite having a therapeutic trough level because the tissue level of anti-TNF may be insufficient to neutralize local inflammatory burden [13-15]. It is possible that a heightened inflammatory burden and local tissue inflammation characterized by high levels of inflammatory cytokines including IL-12/23 and others may serve as a sink for medication, potentially demanding increased drug dosages to achieve the desired therapeutic outcomes, similar to that observed with anti-TNF agents. In addition, presumed differences in pharmacokinetics in different individuals may also contribute to rapid drug clearance and the changes observed [13-15]. Although pediatric pharmacokinetics studies are lacking, a prospective study is needed to define appropriate ustekinumab dosing, interval, and trough levels in management of pediatric UC.
In addition to clinical remission, we also observed higher rates of endoscopic healing in our patient cohort. It has been previously demonstrated that those who achieve this latter outcome have lower rates of retreatment with steroids, decreased rates of colorectal dysplasia and cancer as well as decreased colectomy rates as compared to patients who only achieve clinical remission [16,17]. Our UC patients had high likelihood of colectomy prior to ustekinumab induction based upon their extensive disease, elevated inflammatory markers and prior steroid and anti-TNF use [18-20]. However, we were able to avoid colectomy in 90% of our patients for a year after initiation of ustekinumab therapy. FCP is a good marker of mucosal healing and nearly all of our patients had substantial reductions in their FCP levels when compared to baseline. In our study, endoscopic healing was achieved in 86% of patients who had a follow-up colonoscopy. This response to ustekinumab was encouraging to see and was higher compared to other studies [9,16]. The factors that can contribute to favorable outcomes and mucosal healing include close follow-up with proactive disease monitoring, and early dose escalation with treat to target approach in order to achieve desired outcomes.
Major limitations of our study were small sample size and retrospective study design. Due to this limitation, our study mainly focused on providing a descriptive analysis rather than comparative analysis. We were also not able to measure fully the effect of more than one prior biologic therapy failure on treatment outcomes. In addition, the ustekinumab dosing regimens were not standardized for all patients and were based on the clinical response and severity of symptoms.
Overall, outcomes of response to ustekinumab including clinical, biochemical, and endoscopic healing were very encouraging in our anti-TNF refractory pediatric UC patients. More prospective data on the role of ustekinumab is needed to better understand its role in this complex patient population.
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability Statement
The data produced in this article cannot be shared publicly due to the privacy of the study participants. The de-identified data will be shared upon request to the corresponding author.
Author Contributions
Conceptualization: all authors. Data curation: Riaz MS, Rehman R, Esharif D. Formal analysis; Investigation; Methodology; Project administration; Resources: all authors. Software: Has P. Supervision; Validation; Visualization: all authors. Writing – original draft: Rehman R, Riaz MS. Writing – review & editing: Rehman R, Herzlinger M, Shapiro J, Subedi S. Approval of final manuscript: all authors.