Experience of patients with inflammatory bowel disease in using a home fecal calprotectin test as an objective reported outcome for self-monitoring
Article information
Abstract
Background/Aims
Fecal calprotectin (fC) level is a predictive marker of mucosal healing for patients with inflammatory bowel disease (IBD). Home fC tests are now available. We evaluated the performance of the smartphone-based IBDoc home testing system in patients with IBD and obtained their feedback as an objective patient-reported outcome.
Methods
This prospective study enrolled consecutive patients with IBD in clinical remission. fC in the same stool sample was assessed by using both the laboratory test (Quantum Blue calprotectin test) and home test (IBDoc). The correlation between the 2 tests was analyzed using the Pearson method. In addition, the patients were asked to fill a questionnaire based on their experience.
Results
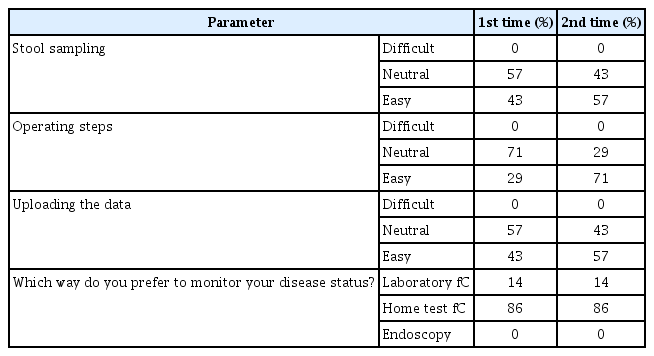
Fifty-one patients with IBD (68 tests and 49 questionnaires) were included. The correlation between Quantum Blue test and IBDoc was good (r=0.776, P<0.0001). After the test, 56% patients found IBDoc easy to perform, and 96% were satisfied with it. Thirty-nine patients (80%) had a strong (>70%) probability to use it for future monitoring if the price was acceptable. By using 250 μg/g as the cutoff, the agreement between home test and laboratory results was 80%, and by using 600 μg/g as the cutoff, the agreement increased to 92%.
Conclusions
The correlation between the laboratory and home tests was good. Most patients found the home test to be feasible and easy to use and preferred it over laboratory test and endoscopy for monitoring. Therefore, the home test could be used as an objective patient-reported outcome.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic inflammatory and destructive disease of the gastrointestinal tract. Chronic inflammation causes ulcerations, stricture formations, and perforations and is a risk factor for dysplasia and cancer [1]. To reduce these longstanding complications, the current treatment goal for patients with IBD is mucosal healing. A treatto-target approach and close monitoring of disease status improve the outcomes for these patients [2].
The gold standard for monitoring patients with IBD is endoscopy, which is time consuming, expensive, and invasive. In addition, pre-procedural bowel cleansing is uncomfortable and inconvenient. Serological biomarkers, such as ESR and CRP, have been widely used as noninvasive parameters for IBD. However, they have insufficient sensitivity and specificity for intestinal inflammation [3,4]. Fecal calprotectin (fC) is correlated with the endoscopic disease activity in patients with IBD; it has been accepted as a noninvasive biomarker for disease activity monitoring [5-7]. There are several laboratory kits for fC measurement such as Quantum Blue® calprotectin, EliA™ calprotectin, and RIDASCREEN® calprotectin. Comparison between these kits showed the accuracy are comparable [8].
For fC assessment, patients have to collect their stool samples, refrigerate them, and send them to the laboratory for further analysis. Depending on the methods and volume of the laboratory, the fC results are sent to the physicians within days to weeks of receiving the stool sample; the patients can know the results only after the physicians informs them about the results. In such situations, the barriers for using fC as a biomarker to monitor disease activity may include the patients’ willingness to collect and bring the samples to the hospital as well as the cost of the test.
Home fC test has become available in the recent years [9]. Studies comparing the accuracy between the laboratory results and patient-evaluated home test results have shown good correlation [10-12]. Home fC testing can eliminate the barriers because patients do not need to store and send the samples. In addition, the cost of maintaining laboratory manpower and equipment can be avoided, decreasing the overall cost.
E-health, or the web-guided patient self-management, is a promising strategy, which could help improve patient compliance and quality of life and decrease depression and anxiety [13]. A smartphone-based home fC test can become an objective marker for e-health. However, no study has evaluated factors related to patient performance and feedback about using the home fC test as an objective marker to monitor their own disease activity. Therefore, we evaluated the patients’ performance by comparing results of the smartphone-based IBDoc home testing system with those of traditional, laboratory-based methods. We also invited the patients to provide their experiences and feedback about using the home fC test through questionnaires to assess patients’ willingness to use this noninvasive test.
METHODS
1. Study Population
This was a prospective study. The study protocol and all amendments were approved by the Ethics Review Board of the National Taiwan University Hospital (IRB No. 201603072RINB) and were conducted in accordance with the Declaration of Helsinki. We enrolled clinically stable patients with IBD, but not those with active symptoms. Patients’ willingness for performing home fC test was enquired. The willing patients’ smartphone would be checked for compatibility with the app of IBDoc. If the smartphone was compatible, the patients were included for further analysis. After providing informed consent, the patients were enrolled consecutively during the outpatient clinic visits. A video about how to execute the test (IBDoc consists of a camera smartphone app and an extraction kit) at home was given to the patients. Next, the patients were given a home test kit, a container for bringing back a stool sample for accuracy correlation analysis with the results of in-house (traditional laboratory test, Quantum Blue® point of care system of Bühlmann Laboratories AG, Schönenbuch, Switzerland) test and the questionnaires. We asked the patients to use the same fecal sample for both home and laboratory tests.
2. Home Test for Fecal Calprotectin
For the home test (IBDoc), a grooved dosing tip dipped 3 to 5 times in the stool was transferred to a container prefilled with 2-mL extraction buffer. The container was shaken several times and left to settle for at least 20 minutes. Via an integrated pipette (valve) 65 μL of extract the supernatant was then applied to the loading port of the lateral flow cassette and left for incubation for 12 minutes. The lateral flow device uses the lateral flow of fluid through a nitrocellulose membrane containing antibodies against calprotectin at a control line and a test line. The sample window contains dried colloid gold-labeled anti-calprotectin antibodies and antibodies against the control antigen. The immune complexes, calprotectin–gold-labeled anticalprotectin antibody and control antigen–control antibody, would diffuse across the membrane and bind to the test and control lines, respectively. The patients then scanned the results shown in the window by the camera setting in the app; then, the results were calculated and sent to the server in European Union. The patients could read the results directly from their smartphone with a colored range as well as quantitative results (calprotectin level in μg/g). The physician could also get an email with a link to the patient’s results. The IBDoc test’s lower and upper limits of detection of fC are 30 and 1,000 μg/g, respectively. The color-coded traffic light categories were green, <250 μg/g; yellow, 250–600 μg/g; and red, >600 μg/g.
The duration of the home test and the time of bringing samples back to the laboratory was within 1 week, and the samples were kept in the refrigerator before they were sent back. After they performed the home tests, the patients self-filled the questionnaires, and the samples for laboratory test and brought them back to study nurses. Some of the patients, when needed in a clinical situation, were asked to do a repeat home test during the study period.
3. Laboratory Test for Fecal Calprotectin
Quantum Blue® fCAL high range point of care system of Bühlmann Laboratories AG was performed following the protocol described before [6]. The laboratory technicians were blinded from the IBDoc test results. The lower and upper limits of the Quantum Blue® calprotectin test were 100 and 1,800 μg/g, respectively.
4. Content of Questionnaires
Patients were asked about their age, sex, and highest level of education. Their disease status; drug compliance; and their experience, expectation, and preference of using IBDoc were also evaluated. Because the experience of using smartphone might influence the results, their experience with using smartphones was also assessed.
5. Statistical Analysis
The correlation between the IBDoc and Quantum Blue® calprotectin test was calculated using Spearman rank correlation (r), where levels below and above the detection limits for the IBDoc test were equalized to 30 and 1,000 μg/g, respectively. In addition, for the Quantum Blue® calprotectin test, the results below and above the detection limits were equalized to 100 and 1,800 μg/g, respectively. These analyses were performed using Microsoft Excel version 16.0 (Microsoft, Redmond, WA, USA). For comparing the patients clinicodemographic characteristics between the groups that responded to home fC test, chi-square test was performed by SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). A P-value of <0.05 was considered significant.
RESULTS
1. Clinical Characteristics of the Patients
From June to July 2016, 51 patients were enrolled in the study. Of them, 27 were diagnosed with UC, 23 with CD, and 1 with indeterminate IBD. Their clinicodemographic characteristics, including sex, age, disease extent, and behavior according to the Montreal classification, are listed in Table 1. Approximately 60% of the patients were younger than 40 years, and the mean age of all was 38.4 years. The male-to-female ratio was 2:1.
Over the study period, 36 patients performed the home test once, 13 performed it 2 times, and 2 performed it 3 times. In total, 68 results of the home fC test and simultaneous laboratory data were collected. Furthermore, 49 of 51 patients (96.1%) successfully completed the IBDoc analysis by themselves and completed the questionnaires. The other 2 performed the test but could not upload the results; 1 of them uploaded with help of the laboratory assistant. Forty-two patients replied once for the questionnaire, and 7 replied 2 times. We pooled the first or only responses for the analysis. We also compared the first- and second-time experience in the 7 patients who responded 2 times.
2. Background Information of the Patients
As shown in Table 2, 80% of the patients claimed that they had good drug compliance, which was defined as missing regular medication dosage less than 10%. Most patients had college (80%) level education. Only 2 patients (4%) had used the smartphone for less than 3 years.
3. The Assessment of Home Test Fecal Calprotectin Operation
Three-fourths of patients understood the meaning of fC as well as the relationship between fC and disease status (Table 3). Before using IBDoc, 6 patients (12%) thought it would be difficult to perform; however, after use, only 2 patients (4%) felt that it was difficult to manage. Up to 90% of patients thought that it might be easier to operate IBDoc the second time onwards, and 40 (82%) believed that with no more than 2 times of practice, they could handle IBDoc. Furthermore, 96% of the patients were satisfied with the home test, and 39 (80%) indicated a strong (>70%) probability of using it if the price was approximately US dollars $20.
We next investigated which step of the home test procedure could preclude the patients from successfully performing the test. In the questionnaire, we asked the patients to grade their experiences while collecting the stool specimen, executing the test, and uploading the results using the smartphone. As shown by Table 4, stool sample collection, procedure execution, and data upload was easy versus difficult as 57% versus 8%, 65% versus 6%, and 69% versus 6%, for the patients, respectively. Moreover, 71% of patients preferred the home test method monitor their disease condition, whereas 27% preferred collecting samples and sending them to the laboratory and 2% preferred endoscopy.
When compared the patients’ clinicodemographic characteristics, such as age, level of education, and experience with using smartphone, we found that before using the home test, highly educated patients were more likely to choose “easy to perform” (P<0.037). There is also a trend that younger patients were more likely to choose easier to perform (P=0.064). However, after the actually practice, there was no any clinicodemographic characteristics affecting the degree of difficulty.
On comparing the first- and second-time experiences of the 7 patients who performed the home test 2 times (Table 5), the patients found all 3 steps to be much easier when performed the second time, with executing the procedure getting the most difference (first time, 29% felt easy; second time, 71% felt easy). Moreover, 86% of these 7 patients preferred to monitor their disease activity using the home fC test, but none of them preferred endoscopy.
4. The Correlation between Home and Laboratory Test of Fecal Calprotectin
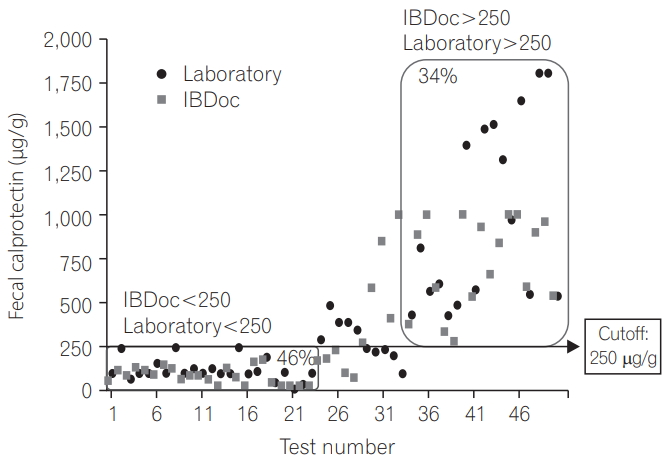
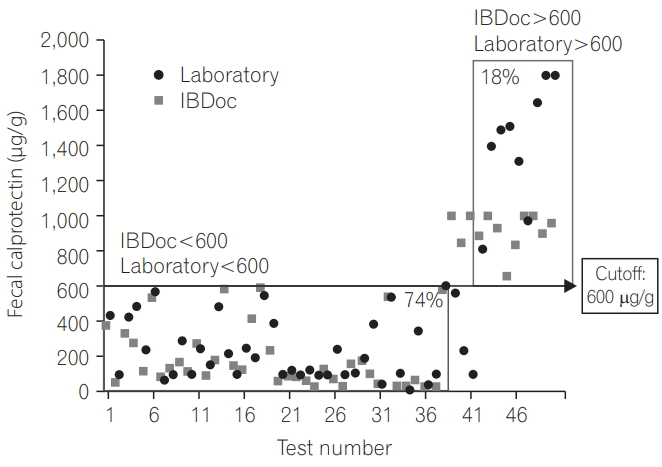
We analyzed the correlation between the home and laboratory test results and found that the correlation was good irrespective of whether we used the 50 test results (r=0.783, P<0.0001) (Fig. 1) or pooled the 68 test results from 51 patients (r=0.776, P<0.0001) (Fig. 2). By using 250 μg/g as the cutoff, the agreement was 80% between the home test and laboratory results (Fig. 3); by using 600 μg/g as the cutoff, the agreement increased to 92% (Fig. 4).
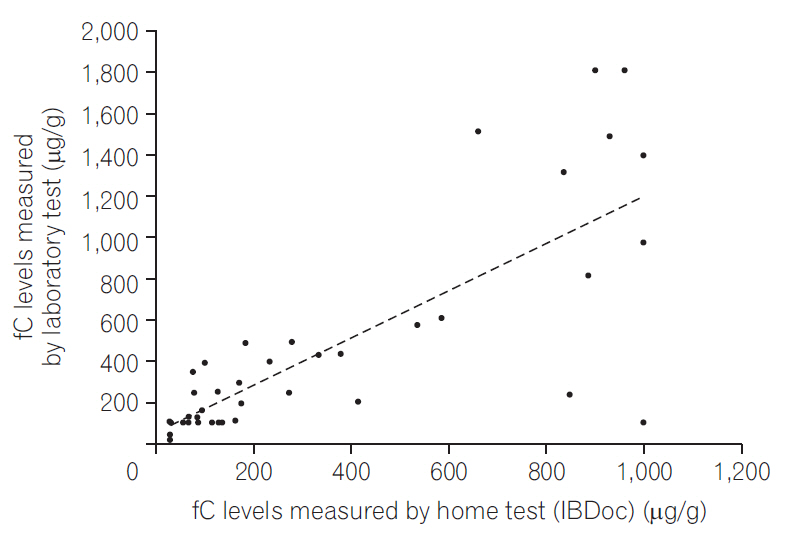
Correlation between the home and laboratory fecal calprotectin (fC) test results (68 tests from 51 patients).
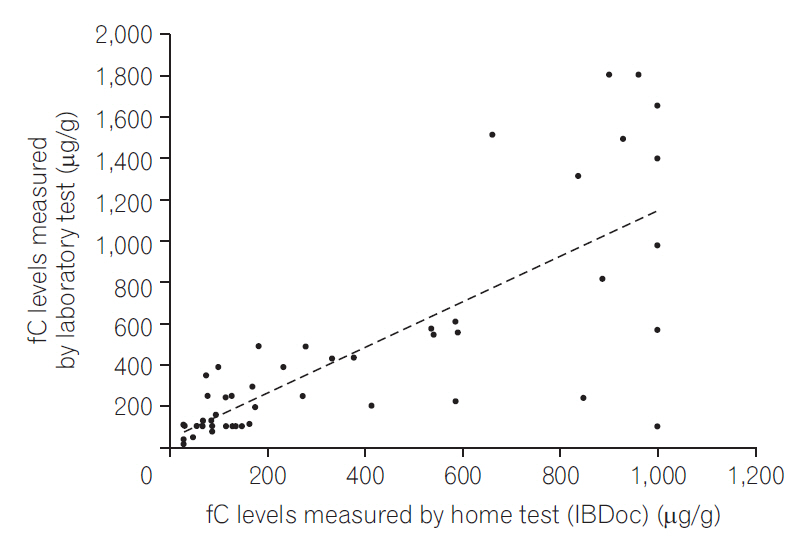
Correlation between the home and laboratory fecal calprotectin (fC) test results (51 tests from 51 patients).
DISCUSSION
Our results showed that patients understood the meaning of fC and that they accepted it as the objective marker that they can handle by themselves at home. Most patients even preferred the home fC test over the laboratory test and endoscopy as their monitoring method. Physician as well as patients might be concerned about the correlation between home fC test, which is handled by the patients themselves, and the laboratory test, which is performed by trained staff. Consistent with previous reports [10-12], the correlation between the 2 in our study was very good.
The cutoff of 250 μg/g (green light on IBDoc) resulted in an agreement of 80% between the home test and laboratory results, but on increasing the cutoff to 600 μg/g (red light IBDoc), the agreement also increased to 92%. Although no universal cutoff for fC has been clinically recognized, several studies have shown that for predicting endoscopic activity in patients with IBD, the optimal cutoff is between 150 and 250 μg/g [5,6,14-18]. An fC of >250 μg/g has been shown to be related to large ulcers on endoscopy [5]. Furthermore, 250 μg/g is an appropriate cutoff for minimizing the variation once the sample has been frozen [18]. Therefore, we set the green light to indicate 250 μg/g. Moreover, we set the red light to indicate 600 μg/g because values of 500 to 600 μg/g nearly guarantee pathology findings [19], to consequently alert the patients to seek further medical treatment.
Although the agreement between the home fC test and ELISA-based results was poor at fC of >500 μg/g, as reconfirmed by another method [10], we noted good agreement (92%) between the results of the home test and Quantum Blue® calprotectin test, which should encourage patients to use the home test.
The app language is English, the second language for our patients. To minimize this possible language barrier, we showed them a video demonstrating how to handle the whole process after enrollment. Thus, despite the potential language barrier, we obtained very positive response from our patients. With such positive response from patients and the good correlation, we conclude that the home fC test can be integrated into the e-health as an objective patient-reported outcome for patients with IBD. However, the patients enrolled into the study were also had higher willingness to perform home fC, that might result to over-estimate the feasibility and patient satisfaction of the home fC test. The acceptance of home fC in real clinical practice remains to be observed.
Several issues require improvement to widen the coverage of the home test. First, not all mobile phones are compatible with the app. Consequently, a few consenting patients unable to download the app and therefore could not be enrolled. Second, the test price and availability may limit its usage. Currently, the home fC test is not widely available. Patients who were enrolled in this study and wanted to continue to use the home test for self-monitoring were unable to do so because the kit is still not available in the Taiwanese market. The kit price might become another major factor affecting the usage. Because of the reduction in workforce required for processing a sample for the fC test, the price of the test may perhaps be decreased to an affordable range, enabling its integrations as an objective outcome indicator self-managed by patients.
Patients require practice to use the home test. Our patient feedback revealed improved sample collection, test execution, and data upload when performing the test the second time. Notably, many patients felt that after only 1 or 2 uses, they could handle the home test without a problem, thereby highlighting the ease of use.
A growing body of evidence suggests that a treat-to-target approach is highly valuable in IBD and leads to better outcomes [20]. However, this approach requires close patient monitoring. This is difficult to achieve with the traditional management approach in IBD because most patients are typically evaluated every 3 to 6 months in an outpatient setting. With the advancements in the Internet and related mobile technologies, e-health is becoming an essential and practical tool for patients’ outcome [21]. E-health could help educate and support patients, improve drug compliance and disease monitoring, and improve outcome based on patient needs and availability, potentially independent of the availability of health care practitioner.
We believe that e-health is particularly crucial for patients with IBD compared with those with other chronic diseases because of IBD’s relapsing–remitting nature. Episodic evaluation according to the traditional model, with serial assessment of clinical symptoms, in an outpatient clinic is insufficient because it hampers quick action in case of clinical relapse and impossible in case of subclinical relapse. By closer monitoring, flares can be predicted and treated more effectively, resulting in a continuous response to therapy with better outcomes [22]. Currently, many apps are available for managing the e-health in patients with IBD, but they still lack an objective indicator. Our results demonstrated the reliability and feasibility as well as patient acceptance of a smartphone-based home fC test. This home fC test could be integrated into the apps and become an objective patient-reported outcome to help the patients monitor their own disease activity more proactively.
Notes
FINANCIAL SUPPORT
The IBDoc kits were provided by Abbvie. This work was supported by the Liver Disease Treatment and Prevention Research Foundation.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION
SCW: study design, patient recruitment, data collection, data analysis and manuscript drafting; CCT: patient recruitment, data collection and writing up the draft of the paper; MTW: data collection and writing up the draft of the paper; JMW: patient recruitment, data collection and results discussion.
Acknowledgements
We thank the second Core Laboratory of the Department of Medical Research of the National Taiwan University Hospital for technical assistance.