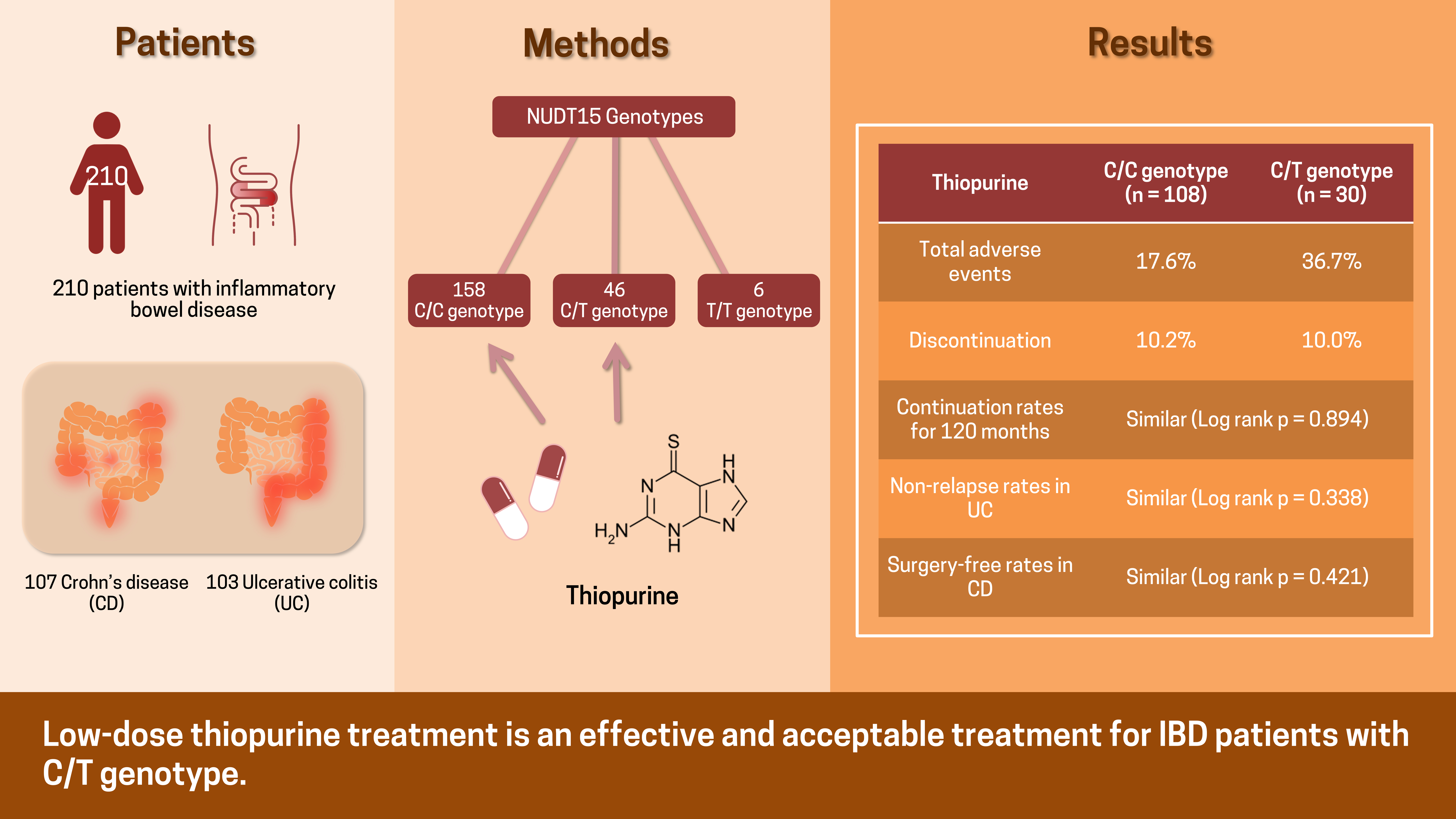
Long-term efficacy and tolerability of dose-adjusted thiopurine treatment in maintaining remission in inflammatory bowel disease patients with NUDT15 heterozygosity
Article information
Abstract
Background/Aims
Thiopurines are key drugs for inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD). Recently, NUDT15 polymorphism (R139C, c.415C > T) has been shown to be associated with thiopurine-induced adverse events in Asian populations. In patients with the C/T genotype, low-dose thiopurine treatment is recommended, but its long-term efficacy and tolerability remain unclear. This study aimed to uncover the long-term efficacy and appropriate dosage of thiopurine for IBD patients with the C/T genotype.
Methods
A total of 210 patients with IBD (103 UC and 107 CD) determined to have NUDT15 R139C variants were enrolled. Clinical data were retrospectively reviewed from medical records.
Results
Of 46 patients (21.9%) with the C/T genotype, 30 patients (65.2%) were treated with thiopurines. Three of whom (10.0%) discontinued thiopurine treatment due to adverse events and 27 of whom continued. The median maintenance dosage of 6-mercaptopurine was 0.25 mg/kg/day (range, 0.19–0.36 mg/kg/day), and 6-thioguanine nucleotides level was 230 (104–298) pmol/8 × 108 red blood cells. Cumulative thiopurine continuation rates for 120 months for patients with the C/C and C/T genotypes were not significantly different (P = 0.895). Cumulative non-relapse rates in the patients with UC treated with thiopurine monotherapy and surgery-free rates in CD patients treated with combination therapy (thiopurines and anti-tumor necrosis factor-α agents) for maintenance remission were not significantly different at 60 months (C/C vs. C/T, P = 0.339 and P = 0.422, respectively).
Conclusions
Low-dose thiopurine treatment is an effective and acceptable treatment for patients with C/T genotype.
INTRODUCTION
Thiopurines such as 6-mercaptopurine (6-MP) and azathioprine (AZA) are widely used for immunomodulatory therapies in patients with inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD). The major therapeutic benefits of thiopurine are known to be its steroid-sparing effect and maintenance of remission of both UC and CD after induction therapy [1,2]. Furthermore, combination therapy with anti-tumor necrosis factor (TNF)-α agents and thiopurines has been shown to be more effective than either as monotherapy [3,4]. Thus, thiopurines presumably improve the pharmacokinetic efficacy of anti-TNF-α agents by reducing the risk of anti-drug antibody formation that leads to a loss of response to anti-TNF-α agents [5,6]. Their additive effect is well defined in CD compared with that in UC [3,5,6].
Although thiopurines are one of the key drugs for patients with IBD, adverse events (AEs) often develop during thiopurine therapy. AEs of thiopurines are divided into dose-independent events, such as allergic reactions, and pharmacologically explainable dose-dependent events [7]. Dose-dependent AEs, such as myelotoxicity and hepatotoxicity, are causing by metabolites of thiopurines, which include active metabolites such as 6-thioguanine nucleotides (6-TGN) and inactive catabolites such as 6-methylmercaptopurine [8].
Recently, a non-synonymous single nucleotide polymorphism (SNP), p.Arg139Cys, in nucleoside diphosphate-linked moiety X-type motif 15 (NUDT15 R139C, c.415C > T) was strongly associated with thiopurine-induced early leukopenia in Koreans [9], and a similar association has been reported in Japanese patient with IBD [10,11]. NUDT15 converts the thiopurine active metabolites 6-thioguanine triphosphate (6-TGTP) and 6-thio-deoxyguanine triphosphate (6-TdGTP), which are incorporated into DNA or RNA and lead to immunosuppression, to 6-tioguanine monophosphate and 6-thio-deoxyguanine monophosphate, respectively [12,13]. Patients who are homozygous and heterozygous for p.Arg139Cys (NUDT15 T/T and C/T genotype, respectively) have lower enzyme activity than those homozygous for the wild allele (C/C genotype), and these variants of NUDT15 caused excessive levels of 6-TGTP and 6-TdGTP, resulting in dose-dependent AEs such as thiopurine-induced leukopenia and alopecia [12]. Therefore, genotyping of NUDT15 R139C is considered the best way to predict thiopurine-induced AEs in Asian patients [11,14].
Thiopurines should be contraindicated in patients with the T/T genotype because several studies have reported that almost all of these patients treated with thiopurines develop severe leukocytopenia and alopecia [10,15]. Patients with the C/T genotype also have a higher risk of dose-dependent AEs than patients with the C/C genotype. Discontinuation of treatment or adjustment of the dosage is not uncommonly required for patient with the C/T genotype. It has been suggested that low-dose 6-MP could be used for patients with the C/T genotype [10]. However, the long-term efficacy and safety of thiopurine remain unclear.
In this study, we reviewed the clinical cases of thiopurine treatment at our institution and investigated the efficacy and tolerability of thiopurine treatment in patients with the C/T genotype compared with patients with the C/C genotype. The goal of this study was to uncover the long-term efficacy and appropriate dosage of thiopurine for IBD patients with the C/T genotype.
METHODS
1. Institutional Review Board Approval
The protocol of the present study was approved by the Institutional Review Board of Hirosaki University Graduate School of Medicine (IRB Nos. 2016-083, 2018-162) and the informed consent was waived. This study was performed in accordance with the ethical standards of the WMA Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subject.
2. Participants
We enrolled 210 patients with IBD (103 UC and 107 CD) who attended Hirosaki University Hospital between January 2015 and October 2018, who consented to this study and were evaluated for NUDT15 R139C genotype. Clinical data were collected by medical records from the institution.
3. DNA Isolation and Single Nucleotide Polymorphism Detection
DNA was extracted from whole blood using QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany), performed in accordance with the manufacturer’s protocols. The purity of DNA was determined by evaluating the optical density 260/280 ratio. SNP analysis was performed using a Step One Plus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). SNP NUDT15 R139C (rs116855232) was genotyped by fluorogenic TaqMan SNP technology with the TaqMan Genotyping Master Mix (Applied Biosystems). The primer sequences were as follows: forward, 5’-CCCCTGGACCAGCTTTTCTG-3’; reverse, 3’-CCACCAGATGGTTCAGATCTTCTTTA-5’. The probe sequences were VIC-CTTTTAAACAACACAGTCCC and FAM-AAACAACGCAGTCCC. The reactions were carried out under the following conditions: 10 minutes at 95°C to initiate polymerase activity, 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. A sample without DNA was used as a negative control.
4. Thiopurine Treatment
The dose of AZA was converted to 6-MP equivalent dose dividing by a conversion factor of 2.08 [15]. 6-MP was administered at an initial dosage of 0.2–0.6 mg/kg/day and screening for AEs was conducted about 2 weeks after initiation. When AEs were developed during thiopurine treatment, we decreased the dosage, discontinued the treatment, or changed the thiopurines depending on the AEs. If patients had no AEs, we increased the dosage as required to approximately 0.5 mg/kg/day. The maintenance dosage of thiopurine was defined as the final dosage that patients could continue without AEs. The serum levels of 6-TGN in maintenance therapy were assessed.
In the patients with UC, to evaluate the efficacy of thiopurine for maintenance therapy, the cumulative clinical non-relapse rate (the time from initiation of thiopurine treatment at the maintenance dosage to clinical relapse [partial Mayo score ≥ 2] requiring more intensive treatment) was compared between patients with C/T and C/C genotypes who achieved remission withdrawing from steroids (partial Mayo score of 0 or 1). These patients were treated with thiopurines for maintenance therapy, and patients treated in combination with antiTNF-α agents were excluded. In the patients with CD, cumulative continuous treatment rates (the time from initiation of combination therapy [anti-TNF-α agent and thiopurine] to requirement of surgery or change in medical therapy) were compared between patients with C/T and C/C genotypes. The definition of surgery was CD-related surgery such as intestinal resections for stricture and fistula and drainage of perianal abscess. In patients with UC and CD, the time-course changes of white blood cell (WBC) count and mean corpuscular volume (MCV) were investigated and compared between patients with C/T and C/C genotypes.
5. Statistical Analysis
Patient age, sex, thiopurine use, duration, dosage of thiopurines, 6-TGN levels, and the frequency of each AE were compared between patients with C/C and C/T genotypes using the Mann-Whitney test and chi-square test in univariate analyses. Analyses of cumulative thiopurine continuation rate, clinical non-relapse rate in the patients with UC, and surgery-free rate in patients with CD were performed using Kaplan-Meier survival plots with the log-rank test. P < 0.05 was regarded as statistically significant. All analyses were performed using GraphPad Prism version 8.0.1 (GraphPad Software, La Jolla, CA, USA).
RESULT
1. Frequencies of NUDT15 R139C Variants and Study Flowchart
The characteristics of the patients enrolled in this study are summarized in Table 1. There were 210 patients, of whom 103 were patients with UC and 107 were diagnosed with CD. The frequencies of NUDT15 R139C variants is summarized in Table 2. Of the 210 patients, 158 patients (75.2%) had NUDT15 C/C genotype, 46 patients (21.9%) had the C/T genotype, and 6 patients (2.9%) had the T/T genotype. Flow of the analysis in this study was indicated in Supplementary Fig. 1.

Comparison of thiopurine dosage, time to adverse events (AEs), cumulative continuation rate according to NUDT15 genotypes. (A) Maintenance dosage of 6-mercaptopurine (6-MP) and serum 6-thioguanine nucleotides (6-TGN) levels in patients with C/C and C/T genotypes. (B) Dosage of 6-MP at the time when total AEs was developed are plotted. (C) Time to total AEs after starting thiopurine treatment is plotted in term of NUDT15 genotypes. (D) Cumulative continuation rate of thiopurine for 120 months in patients with C/C and C/T genotypes. Azathioprine doses were converted to a 6-MP dose using a conversion factor of 2.08. Data represents median (interquartile range). aP < 0.05. RBCs, red blood cells.
2. Thiopurine Treatment According to NUDT15 Genotype
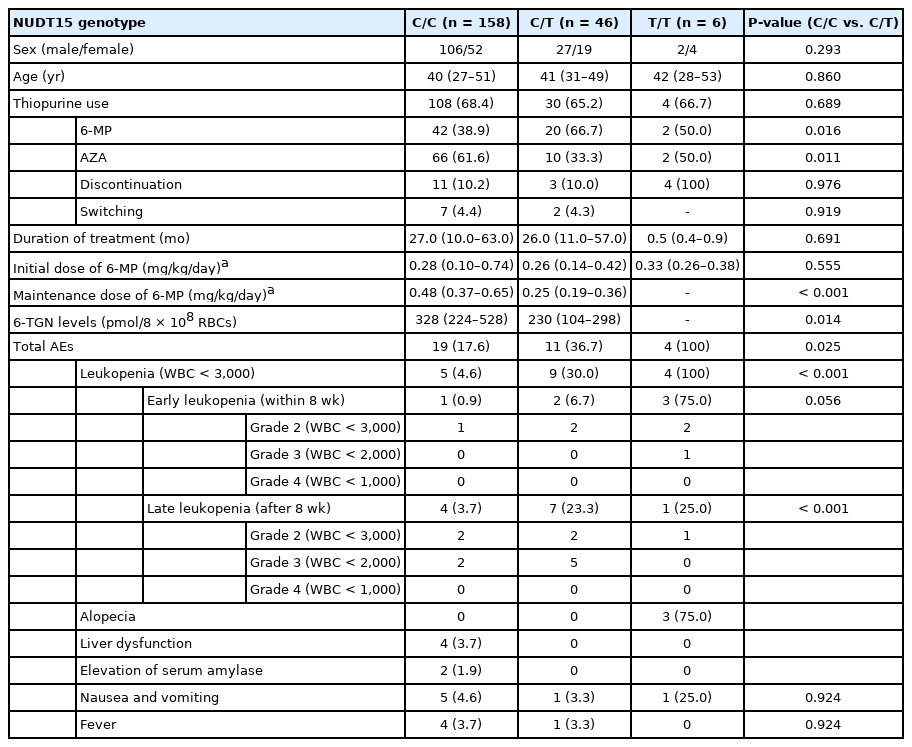
Information about thiopurine treatment, 6-TGN levels and AEs according to the each NUDT15 genotype is summarized in Table 3. The age of patients, sex, and duration of thiopurine treatment were not significantly different between patients with C/C and C/T genotypes. Patients with C/C and C/T genotypes who were treated with thiopurines numbered 108 (68.4%) and 30 (65.2%), respectively (P = 0.689). The percentage of patients with the C/T genotype who received 6-MP (66.7%) was significantly higher than that of patients with the C/C genotype (38.9%, P = 0.016). The median initial dosage of 6-MP was 0.28 mg/kg/day for patients with the C/C genotype and 0.26 mg/kg/day for patients with the C/T patients, with no significant difference (P = 0.555). The median maintenance dosage of 6-MP was 0.48 mg/kg/day for patients with the C/C genotype and 0.25 mg/kg/day for those with the C/T genotype (P < 0.001) (Fig. 1A). The 6-TGN levels at the maintenance dosage of thiopurine treatment were significantly lower in patients with the C/T genotype (230 pmol/8 × 108 red blood cells [RBCs]) than in those with the C/C genotype (328 pmol/8 × 108 RBCs) (P = 0.014) (Fig. 1A).
The percentage of total AEs in patients with C/C and C/T genotypes was 17.6% and 36.7%, respectively (P = 0.025). The development of total leukopenia and late (after 8 weeks) leukopenia was significantly higher in patients with the C/T genotype than in those with C/C genotype (P < 0.001 and P < 0.001, respectively). All patients with the T/T genotype developed leukopenia. The median dosage of 6-MP when total AEs developed were 0.500 mg/kg/day (range, 0.429–0.612 mg/kg/day) for patients with the C/C genotype and 0.462 mg/kg/day (range, 0.401–0.511 mg/kg/day) for patients with the C/T genotype (P = 0.366) (Fig. 1B). The median time to total AEs after the initiation of thiopurine treatment was 18 weeks (range, 4.75–134 weeks) in patients with the C/T genotype and 5 weeks (range, 2–9.5 weeks) in patients with the C/C genotype (P = 0.018) (Fig. 1C). The percentage of patients who required discontinuation of thiopurine treatment (Table 3) and the cumulative continuation rate of thiopurine treatment (Fig. 1D) were not significantly different between patients with C/C and C/T genotypes (P = 0.976 and P = 0.895, respectively).
3. Thiopurine Monotherapy for Maintenance Remission in Patients with UC
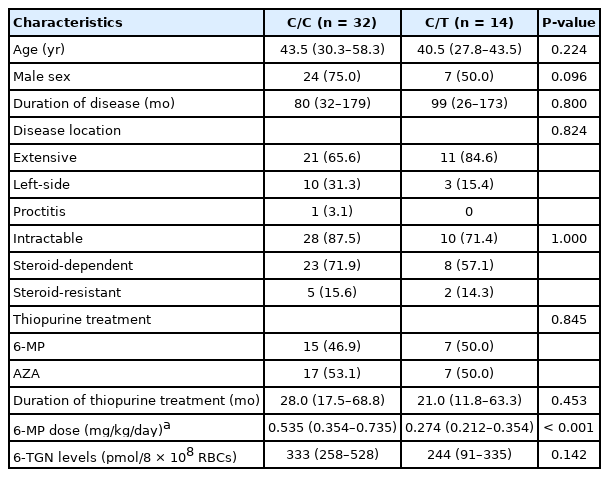
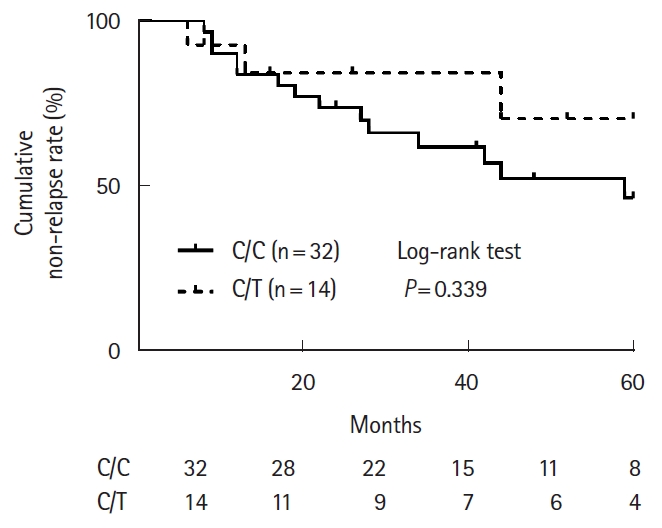
The characteristics of UC patients with C/C and C/T genotype in this analysis are shown in Table 4. These patients were treated with thiopurine without anti-TNF-α agents as maintenance therapy. The 6-MP dosage were significantly higher for patients with the C/C genotype (median, 0.535 mg/kg/day) than for those with the C/T genotype (median, 0.274 mg/kg/day; P < 0.001), but median 6-TGN levels in patients with the C/C and C/T genotypes were 333 pmol/8 × 108 RBCs and 244 pmol/8 × 108 RBCs, respectively, without significant difference (P = 0.142). The cumulative non-relapse rate for 60 months after initiation of thiopurine monotherapy was not significantly different between patients with C/C and C/T genotypes (P = 0.339) (Fig. 2).
4. Thiopurine Therapy Combined with Anti-TNF-α Agents for Maintenance Remission in Patients with CD
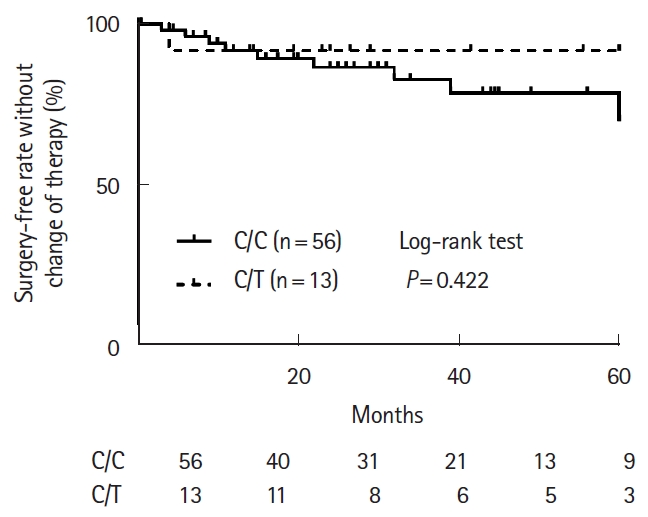
The characteristics of CD patients with C/C and C/T genotypes in this analysis are shown in Table 5. Similar to the trend observed in UC patients, the percentage of the patients treated with 6-MP was significantly higher for patients with the C/T genotype than for those with the C/C genotype (P = 0.012). The 6-MP dosage was significantly higher for patients with the C/C genotype (median, 0.48 mg/kg/day) than for those with the C/T genotype (median, 0.19 mg/kg/day; P < 0.001), but 6-TGN levels were not significantly different (P = 0.17). The cumulative surgery-free rate without change of therapy for 60 months after initiation of thiopurine combination therapy were not significantly different between patients with the C/C and C/T genotypes (P = 0.422) (Fig. 3).
5. Time-Course Changes of WBC Count and MCV
We investigated the changes of WBC count and MCV in the patients with the C/C and C/T genotypes. We defined the WBC count at start of the treatment with thiopurine as 100%. The WBC counts of both groups gradually decreased after start of the treatment with thiopurine. The WBC counts at 3 months in the C/T genotype and in the C/C genotype decreased significantly from that at the baseline (P < 0.001 and P < 0.001, respectively) (Fig. 4A). The WBC counts in the C/T genotype were significantly lower than that in the C/C genotype at 48, 60, 72, 96 months after start of the treatment with thiopurine (P = 0.039, P = 0.049, P = 0.008, P = 0.039, respectively) (Fig. 4A). MCV also gradually increased after initiation of thiopurine. The MCV at 6 months in the C/T genotype and at 3 months in the C/C genotype increased significantly from that at the baseline (P = 0.002 and P < 0.001, respectively) (Fig. 4B). The MCV in the C/T genotype were significantly higher than that in the C/C genotype at 60, 72 months after start of the treatment with thiopurine (P = 0.016 and P = 0.033, respectively) (Fig. 4B).
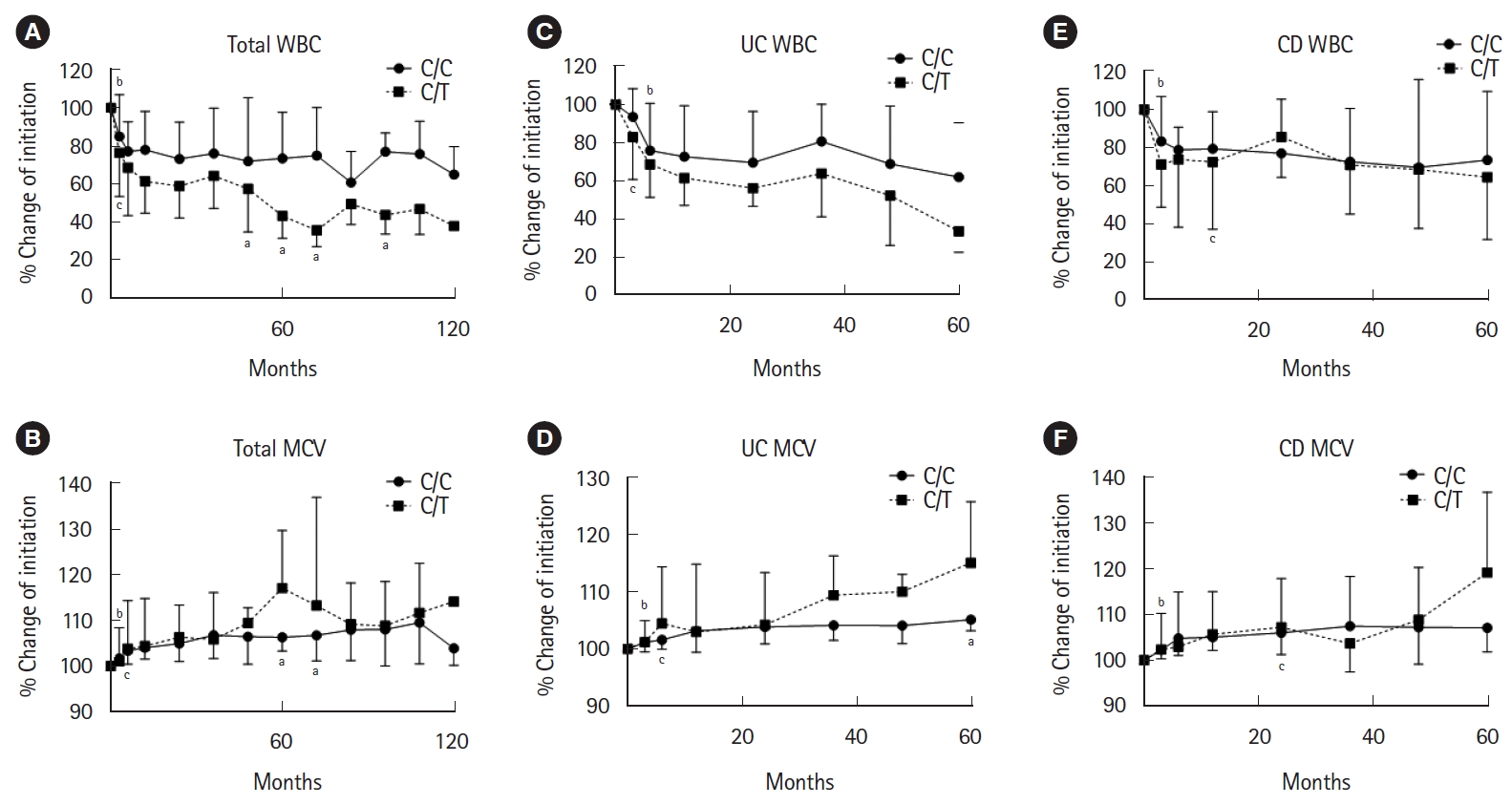
Time-course of change in white blood cell (WBC) count (A, C, E) or mean corpuscular volume (MCV) (B, D, F) for 120 or 60 months in patients with NUDT15 C/C and C/T genotypes. Data represents median (interquartile range). aStatistically significant difference between the C/C and C/T genotypes (P < 0.05). Statistically significant difference from the start of thiopurine therapy (bC/C genotype; cC/T genotype, P < 0.05). UC, ulcerative colitis; CD, Crohn’s disease.
Next, we analyzed the changes of WBC count and MCV for UC or CD individually in the patients with the C/C and C/T genotypes. The WBC counts of both CD and UC gradually decreased after start of the treatment with thiopurine. In UC patients, the WBC counts at 3 months in the C/T genotype and at 6 months in the C/C genotype decreased significantly from that at the baseline (P = 0.004 and P < 0.001, respectively) (Fig. 4C). There were no differences of the change in WBC counts between the C/C and C/T genotype (Fig. 4C). The MCV at 3 months in the C/T genotype and at 6 months in the C/C genotype increased significantly from that at the baseline (P < 0.005 and P = 0.01, respectively) (Fig. 4D). The MCV in the C/T genotype was significantly higher than that in the C/C genotype at 60 months (P = 0.048). In CD patients, the WBC counts at 3 months in the C/C genotype and at 12 months in the C/T genotype decreased significantly from that at the baseline (P < 0.001 and P = 0.002, respectively) (Fig. 4E). There were no significant differences in increase of MCV between the C/C and C/T genotypes. The MCV at 3 months in the C/C genotype and at 24 months in the C/T genotype increased significantly from that at the baseline (P < 0.001 and P = 0.002, respectively) (Fig. 4F). There were no differences of the change in WBC count and MCV between the C/C and C/T genotype (Fig. 4E and F).
DISCUSSION
Genotyping of NUDT15 R139C has been shown to be the best way to predict thiopurine-induced AEs in Asian populations with IBD [11,16]. Patients with NUDT15 C/T and T/T genotypes have a high risk of developing thiopurine-induced AEs because these variants of NUDT15 cause excessive levels of thiopurine active metabolites (such as 6-TdGTP and 6-TGTP including in 6-TGN) that are incorporated into DNA or RNA, resulting in dose-dependent AEs [12]. In patients with the C/T genotype, low-dose 6-MP (0.2–0.3 mg/kg/day) may be acceptable, but its long-term efficacy and tolerability remain unclear.
The frequency of NUDT15 polymorphism in this study was similar to that reported in previous studies in the East Asian population [9,12,14]. The development of AEs was more frequent in patients with C/T and T/T genotypes than in those with the C/C genotype. Patients with the T/T genotype should avoid thiopurine treatment because almost all of these patients experience severe AEs such as early severe leukopenia. In this study, one of the 4 patients with the T/T genotype developed late leukopenia. Low initiative dose of thiopurine, the poor patient compliance due to nausea, and poor absorption due to short bowel syndromes by multiple CD-related surgeries was thought to affect the results. Although the development of AEs in patients with the C/T genotype was more frequent than in patients with the C/C genotype, the frequency of thiopurine discontinuation or long-term continuation of thiopurine treatment was not significantly different. AEs observed in patients with the C/T genotype were mostly alleviated by reducing the thiopurine dosage. Compared with AZA, 6-MP is more useful for these patients because finer dose adjustment is possible due to its fine grain. Careful monitoring of WBC count is needed to avoid severe AEs as well as dose adjustment [17]. In the present study, the decrease rate in WBC count from baseline did not shown significant differences between the C/T and C/C genotype in the early phase. However, the decrease rate of WBC count in the C/T genotype was significantly higher than that in the C/C genotype in the late phase. Therefore, more careful long-term monitoring of WBC count with a dose adjustment is needed in case of C/T genotype.
Thiopurine is more effective in maintaining mucosal healing in UC than in CD [18]. Thiopurine is the key drug for maintaining remission of UC, and at our institution, thiopurine is mainly used in combination with mesalazine. In this study, the cumulative non-relapse rates of UC patients with C/C and C/T genotypes were compared to assess the long-term efficacy of thiopurine for maintenance therapy. The results showed that the non-relapse rate of patients with the C/T genotype was not significantly different from that of patients with the C/C genotype.
In CD patients, combination therapy with thiopurine and anti-TNF-α agent showed more favorable outcomes than antiTNF-α monotherapy [2,3]. Previous reports have shown that only about 20% of patients treated with thiopurine sustain mucosal healing after 2 years [18]. At our institution, thiopurine, in combination with infliximab (IFX) is mainly used to maintain remission in patients with CD. We calculated long-term cumulative surgery-free rates without change of therapy between CD patients with C/C and C/T genotypes treated with combination therapy and observed no significant difference between the 2 groups. These results suggest that low-dose thiopurine treatment for patients with the C/T genotype can be continued and is effective for maintenance therapy.
The effectiveness of thiopurine is considered to be associated with serum 6-TGN levels. In our study, the overall analysis including both CD and UC showed a significant difference of 6-TGN levels between the C/C and C/T genotype. However, the significant difference was disappeared in the individual analysis of UC or CD. The decrease of the number of patients in the individual analysis was thought to affect the result. Previous studies have reported that 6-TGN levels correlated with the therapeutic efficacy of thiopurine are 230–450 pmol/8 × 108 RBCs [19,20]. In this study, the ranges of 6-TGN levels in UC patients with C/C and C/T genotypes exceeded 230 pmol/8 × 108 RBCs and were not significantly different between 2 groups. In addition, maintaining 6-TGN levels above 125 pmol/8 × 108 is adequate for achieving therapeutic levels of IFX in the patients treated with combination therapy (IFX and AZA or 6-MP) [21]. In this study, 6-TGN levels in CD patients with C/C and C/T genotypes were above 125 pmol/8 × 108 and were not significantly different between the 2 groups. These results suggest that even low-dose thiopurine can achieve a therapeutic range of 6-TGN levels and expected efficacy in patients with the C/T genotype. However, levels of IFX were not assessed in our study, and it is still unclear whether serum IFX levels in patients treated with thiopurine are different between the C/T and C/C genotypes.
MCV is thought to be one of surrogate indicators of 6-TGN concentration [22]. MCV gradually increased after start of the treatment with thiopurine in both groups. While the increase of MCV in the C/T genotype was significantly higher than that in the C/C genotype at the several points after thiopurine therapy, that was not stable. MCV is known to be influenced by various nutritional factors such as iron deficiency and concomitant drugs [22]. In the present study, the number of patients with iron concentration available was small and further studies would be required to clarify this point.
As for fine adjustment of dosage, the median maintenance dosage of thiopurine for all patients with the C/T genotype was 0.25 mg/kg/day (as 6-MP dosage), and median 6-TGN level was 230 pmol/8 × 108 RBCs. Compared with those in patients with the C/C genotype, both parameters were significantly low. However, the development of all AEs was more frequent in patients with the C/T genotype. These data suggest that the index of 6-TGN levels itself might not be an appropriate marker for predicting thiopurine-induced AEs in patients with the C/T genotype. Other appropriate indicators for 6-TGN levels should be identified that reflect thiopurine metabolites. In fact, a previous study reported that 6-TGN levels are not associated with NUDT15 R139C related thiopurine-induced leukopenia in Japanese patients with IBD [15]. Recently, measuring DNA-incorporated thioguanine metabolites (i.e., 6-TGTP and 6-TdGTP) in WBCs has been shown to be a better predictive marker for thiopurine-induced leukopenia than 6-TGN levels, because they are more directly related to DNA damage [12,23]. Measurements of DNA-incorporated thioguanine might be a predictive factor for the development of AEs and therapeutic effectiveness in patients with NUDT15 variants. Further studies are needed to confirm this.
There are several limitations in this study. First, this was a retrospective, small and single-center cohort study. Recent studies indicate that the prevalence of NUDT15 C/T genotype is about 20% in Japanese patients with IBD [11,15]. It is not easy to monitor the patients with NUDT15 C/T genotype who receive thiopurine over the long time. Though the sample size of is small, the present study is thought to contain important meanings. Second, our analysis included patients who had started thiopurine treatment before investigating NUDT15 R139C variants, which may result in a bias in the initial dosage of thiopurine and frequency of AEs. Further analyses such as prospective or multicenter analyses should be performed to investigate the efficacy of thiopurine treatment in the patient with C/T genotype.
In conclusion, our study shows that low-dose thiopurine treatment for patients with the NUDT15 C/T genotype can be continued without severe AEs by fine adjustment of the dosage and is as effectiveness as maintenance therapy with standard dosage of thiopurine for patients with the C/C genotype. Our data suggest that the dosage adjustment of thiopurine as determined by NUDT15 variant provides favorable effects to maintain remission in IBD.
Notes
Funding Source
This research was supported in part by Japan Agency for Medical Research and Development (AMED) under Grant Number JP18kk0305002 to Kakuta Y.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contribution
Conceptualization: Maeda T, Sakuraba H. Data curation and acquisition: Maeda T, Yoshida S. Investigation: Hiraga H, Kikuchi H, Hasui K, Tatsuta T, Chinda D, Mikami T. Methodology: Sakuraba H, Hiraga H, Yoshida S, Kakuta Y. Funding acquisition: Kakuta Y. Supervision: Fukuda S. Writing – original draft: Maeda T. Writing – review & editing: Sakuraba H, Kawaguchi S. Approval of final manuscript: all authors.
Non-Author Contribution
We wish to thank Yoichi Kakuta for providing valuable help and support with the genetic analysis of NUDT15.
Others
We thank all the staff of our hospital and the patient.
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Fig.1. Flow of the analysis in this study. In total, 210 patients with inflammatory bowel disease (IBD) genotyped for NUDT15 R139C were enrolled. Among them, 138 patients with C/C or C/T genotype exposed to thiopurines were analyzed for cumulative thiopurine continuation rate (Fig. 1D). Of them, 46 patients with ulcerative colitis (UC) who received thiopurines on maintenance therapy were analyzed for cumulative non-relapse rate (Table 4, Fig. 2), and 69 patients with Crohn’s disease (CD) receiving combination therapy with anti- tumor necrosis factor (TNF)-α agents were analyzed for cumulative surgery-free rate (Table 5, Fig. 3).
ir-2020-00133-suppl.pdf