Vedolizumab for perianal fistulizing Crohn’s disease: systematic review and meta-analysis
Article information
Abstract
Background/Aims
Perianal fistulas are a debilitating manifestation of Crohn’s disease (CD). Despite the advent of anti-tumor necrosis factor (anti-TNF) therapy, the medical management of fistulizing CD continues to be challenged by unmet needs. We conducted a systematic review and meta-analysis of the effectiveness of vedolizumab for the management of perianal fistulizing CD.
Methods
A search of PubMed, EMBASE and the Cochrane Library was performed from inception to June 2020 for studies reporting rates of perianal fistula healing in CD patients treated with vedolizumab. The primary outcome of interest was complete healing of perianal fistulas and the secondary outcome was partial healing. The pooled fistula healing rates with 95% confidence intervals (CI) were calculated utilizing a random effects model.
Results
A total of 74 studies were initially identified, 4 of which met the inclusion criteria. A total of 198 patients with active perianal fistulas were included, 87% of whom had failed previous anti-TNF therapy. The pooled complete healing rate was 27.6% (95% CI, 18.9%–37.3%) with moderate heterogeneity (I2=49.4%) and the pooled partial healing rate was 34.9% (95% CI, 23.2%–47.7%) with high heterogeneity (I2=67.1%).
Conclusions
In a meta-analysis of 4 studies that included 198 patients with perianal fistulizing CD, the majority of whom had failed previous anti-TNF therapy, vedolizumab treatment led to healing of perianal fistulas in nearly one-third of the patients. The lack of high-quality data and significant study heterogeneity underscores the need for future prospective studies of fistula healing in patients receiving anti-integrin therapy.
INTRODUCTION
Crohn’s disease (CD) is a lifelong inflammatory bowel disease of unknown etiology. CD may involve any part of the gastrointestinal tract and can manifest in distinct clinicopathologic phenotypes including a stricturing, penetrating (fistulizing), or inflammatory (non-stricturing, non-penetrating) phenotype. An estimated 20% to 40% of CD patients develop a fistula in their lifetime [1,2] and at least 77,000 patients are estimated to live with fistulizing CD in the United States [3]. Given the significant morbidity associated with fistulizing disease, this is a substantial complication of CD.
Perianal fistulas are the most common manifestation of fistulizing CD and adequate management frequently requires a combined surgical and medical approach. Anti-tumor necrosis factor α (anti-TNF-α) biologics, particularly infliximab, are widely used for perianal fistulizing CD. In the pivotal placebocontrolled randomized trial of infliximab for fistulizing CD, complete fistula remission within 18 weeks was observed in 13% of patients receiving placebo, 55% of patients receiving 5 mg/kg infliximab every 8 weeks (P=0.001), and 38% of patients receiving 10 mg/kg every 8 weeks (P=0.04) [4]. Similar healing rates were seen in a post-hoc analysis of the pivotal maintenance trial of adalimumab, and both anti-TNF therapies can be augmented with ciprofloxacin [5-7]. However, there is a paucity of randomized, controlled trials evaluating the efficacy of non-TNF biologics for perianal fistulizing disease.
Vedolizumab is a gut-selective α4β7 integrin antibody that is effective for induction and maintenance of remission of CD [8]. It has been shown to be effective in CD patients who are anti-TNF naïve, in those who are primary nonresponders to antiTNFs, and those who develop secondary nonresponse due to mechanistic escape, development of antibodies or drug-related adverse events [9]. There remains uncertainty about the effectiveness of vedolizumab for the management of perianal fistulizing CD. We therefore conducted a systematic review and meta-analysis of the evidence of the effectiveness of vedolizumab for perianal fistulizing CD.
METHODS
This meta-analysis was performed in accordance with the criteria established in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines. This study was registered with the University of York International Prospective Register Of Systematic Reviews (PROSPERO; Registration number CRD42020209930) [10].
1. Search Strategy and Study Selection
Studies were identified by performing a literature search of 3 electronic databases (MEDLINE through PubMed, EMBASE and the Cochrane Central Register of Controlled Trials) with the last search performed in June 2020. The detailed search strategy is outlined in Supplementary Table 1. We attempted to identify additional studies by reviewing the reference list of all included studies and performing a manual search to retrieve other relevant articles that may have been missed with the initial search strategy. There was no language restriction and manuscripts were translated to English if necessary. Authors of manuscripts were contacted when any data were missing in the manuscripts. Two investigators (F.A. and M.O.) screened all titles and abstracts for relevance to the study. The full text of potentially eligible studies was subsequently reviewed by 2 investigators (F.A. and M.O). Disagreements were resolved by consensus or by consulting with a third investigator (A.S.).
2. Inclusion and Exclusion Criteria
Inclusion criteria were: (1) retrospective or prospective, case series (larger than 10 patients), case-control, or cohort studies and clinical trials (including randomized clinical trials); (2) studies including adult patients with CD treated with vedolizumab; (3) studies reporting number of patients with perianal fistulizing disease; and (4) studies reporting fistula-specific outcomes of patients treated with vedolizumab. Exclusion criteria were: (1) studies not reporting numbers of included patients with perianal fistulizing disease; (2) studies not reporting fistula-specific outcomes; (3) reviews, commentaries, surveys; (4) and duplicate studies.
3. Data Extraction
Data from each eligible study were extracted using a standardized data extraction sheet. The extracted data included: (1) study authors, (2) year of publication, (3) setting (location), (4) study type (retrospective or prospective/single-center or multicenter), (5) patient demographics (age, sex), (6) number of patients with active perianal disease at initiation of vedolizumab, (7) number of patients with setons at initiation of vedolizumab, (8) previously failed CD therapies, (9) vedolizumab dosage and frequency, (10) number of patients who had vedolizumab dose escalation, (11) number of patients on concomitant immunomodulator, antibiotic or steroid therapy, (12) definitions of active perianal fistula and fistula healing, (13) time point for assessment of healing, (14) number of patients with partial healing, (15) number of patients with complete healing, and (16) number of patients with recurrent or de novo fistulas during treatment. Due to the nature of this research article not involving individual patients or patient data (meta-analysis), ethical approval was not required or obtained from the University of Chicago.
4. Outcomes and Definitions
The primary aim of this study was to conduct a meta-analysis assessing the rates of complete healing of perianal fistulizing disease in CD patients treated with vedolizumab. A secondary aim was to assess the rates of partial healing of perianal fistulizing disease. Active perianal fistulizing disease was defined by clinical and/or imaging evidence including perianal abscess, perianal external openings with drainage, and/or imaging evidence demonstrating perianal abscess with or without fistula tract formation. Complete healing was defined by investigator-determined complete resolution of perianal pain, fluctuation or drainage with/without imaging evidence in support of healing, without the need for any additional medical and/or surgical therapy directed at the perianal disease [11]. Partial healing was defined as at least 50% reduction from baseline in the number of draining perianal fistulas (of those draining at baseline) [12]. We also collected data on the rates of recurrent or de novo fistula formation during treatment in patients with inactive perianal disease or no history of perianal disease.
5. Assessment of Methodologic Quality
The quality of studies was assessed using the Newcastle-Ottawa Scale (NOS) [13]. Since the majority of included studies were not randomized placebo-controlled trials, we utilized a modified version of the NOS appropriate for our analysis. This tool removes from the NOS the items that relate to comparability between 2 arms and retains items that assess representation and selection of cases as well as ascertainment of exposure and outcome [13]. A point is assigned to each component of the modified scale, with the highest possible score being 6/6. Studies were considered to be high quality if they scored 6/6, moderate quality if they scored 5/6 and low quality if they scored 4/6 or less. The quality of all studies was assessed by 2 investigators (F.A. and M.O.). Since less than 10 studies were included, visual inspection of the funnel plot was used to assess for publication bias [14].
6. Statistical Analysis
The pooled rates were calculated utilizing a random effects model and the Freeman-Tukey arcsine transformation was used [15]. The Cochran Q test and I2 were used to assess heterogeneity of included studies. I2 values of < 25%, 25%–50%, and > 50% were considered to represent low, moderate, and high heterogeneity, respectively [14]. P-values < 0.05 were considered significant and all tests were two-tailed. The study was performed in accordance with the PRISMA recommendations for reporting systematic reviews and meta-analyses. Analysis was conducted using Stata, version 15 (StataCorp., College Station, TX, USA).
RESULTS
1. Search Results
The flow diagram for study selection is depicted in Fig. 1. Overall, 72 studies were identified using our search strategy from database search and 2 additional records were identified through other sources. Eleven items were duplicates and were excluded. Of the remaining 63 studies after duplicate removal, 53 were excluded after screening titles and abstracts. Full text review was then performed on 10 studies using the predefined inclusion and exclusion criteria, after which 4 studies [11,12,16,17] were retained. Two of the 4 studies were randomized, controlled clinical trials (one was a post-hoc exploratory analysis of the pivotal GEMINI 2 randomized, placebo-controlled vedolizumab maintenance trial [16], the other was a randomized trial of 2 vedolizumab dosing regimens; standard dosing versus standard dosing plus an additional dose at week 10 [12,18]). The other 2 studies were retrospective cohort studies [11,17]. Of note, all patients in the study by Feagan et al. [16] received vedolizumab induction therapy, but were then randomized to maintenance with either placebo or vedolizumab. Only outcomes of those who received both vedolizumab induction and maintenance therapy were included in our analysis to allow for appropriate comparisons with the other included studies.
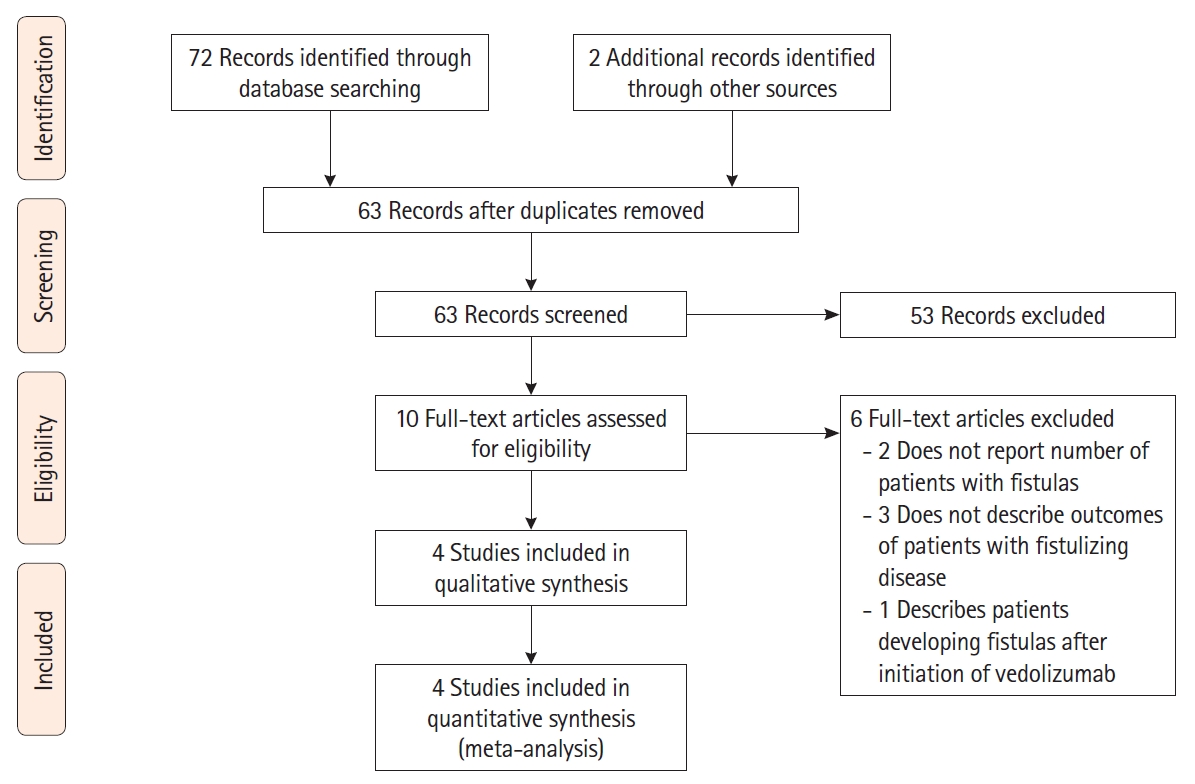
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram depicting the selection process of studies used in the analysis.
All studies were published between 2018 and 2020. All were multicenter studies, 2 of which were conducted internationally and 2 in France. Of note, the study by Pestour et al. was presented at the 13th Congress of the European Crohn’s and Colitis Organisation (ECCO) and published in abstract form [19] and also published as a peer-reviewed Master’s degree thesis document in an open-access web repository utilized by French higher-education institutions (L’archive ouverte pluridisciplinaire [HAL]) [17]. The study by Schwartz et al. was presented at Digestive Disease Week (DDW) 2020 and published in abstract form [18] with the final full results of the clinical trial posted publicly on the European Union Clinical Trials Register; this final version was used for this analysis [12].
2. Patient Population and Study Characteristics
A total of 198 patients with active perianal fistulizing disease were treated with vedolizumab induction and maintenance. Study and patient characteristics are summarized in Table 1. All studies except the study by Pestour et al. [17] reported age and sex of included patients; mean age ranged from 32.6 to 35.1 years and 74 out of 169 patients (44%) were males. The modality for assessment of fistula healing was reported by all studies except the study by Pestour et al. [17]; the remainder of studies used clinical examination to assess for healing, with 2 studies [11,12] using imaging in addition to examination. The clinical trial by Schwartz et al. [12] utilized the most comprehensive assessment for fistula healing, combining clinical examination, magnetic resonance imaging as well as several validated indices for perianal disease activity. Studies varied in their time points for primary outcome assessment from as short as 24 weeks following induction with vedolizumab [17] up to 52 weeks [11,16]. Only 1 study [11] reported the number of patients with setons at the time of induction where 61 out of 102 patients (60%) had setons and only 9 out of 61 (15%) had successful seton removal during follow-up. Two studies [11,16] reported need for surgical intervention directed at the perianal disease during vedolizumab therapy, both considered need for surgery a failure of vedolizumab. In the study by Chapuis-Biron et al., incision and drainage were required in 21 patients and 9 patients required fecal diversion. In the trial by Feagan et al., 3 patients had “fistula surgery” (1 during induction and 2 during maintenance), however the type of surgery was not specified.
All studies reported previous biologic therapies for patients, with the 2 studies [11,17] where all patients had failed prior antiTNF therapy, one study [16] where 19 out of 39 (49%) and another [12] where 22 out of 28 (79%) patients had failed prior anti-TNF therapy. Overall, 87% of patients had failed previous anti-TNF therapy. Reasons for previous failure of anti-TNF therapy were not described in any of the studies.
3. Treatment Characteristics
All studies reported their vedolizumab dosing regimens (Table 2). All utilized standard dosing with vedolizumab 300 mg administered intravenously at weeks 0, 2, 6 (induction) and then every 4 or 8 weeks thereafter (maintenance) and the randomized clinical trial by Schwartz et al. [12] compared this standard regimen to a similar regimen plus an additional dose at week 10. The 2 studies included randomized clinical trials [12,16] did not allow dose escalation if no clinical response was noted, with the other 2 retrospective studies [11,17] allowing dose escalation and reported escalation dosing regimens. In the study by Chapuis-Biron, 62 out of 102 patients (61%) required dose escalation and the study by Pestour et al. did not report the number of patients requiring dose escalation. Three studies [11,12,16] reported the number of patients on concomitant immunomodulator therapy with overall 66 out of 169 patients (39%) on a stable dose of concomitant therapy with an immunomodulator. Two studies reported the number of patients who required any antibiotic therapy throughout the study with 60 out of 141 (43%) patients, however, no details were provided on dosing, type, or duration of antibiotic therapy. Two studies [12,16] reported that 28 out of 67 (42%) patients required concomitant steroid therapy throughout the study, but no details were provided on dosing or duration of therapy.
4. Severity of Perianal Disease
Two of four studies provided descriptive information on the severity and number of perianal fistulas, however these data were not uniform. Feagan et al. reported the number of draining fistulas per patient with 13 patients having 1 draining fistula, 4 with 2 draining fistulas and 1 with 3 or more draining fistulas. Pestour et al. described the severity of perianal disease, with isolated fistulas in most cases (n = 18; 67%), fistulas associated with anal stenosis (n = 4; 15%), fistulas associated with anal ulcers (n = 3; 11%), and fistulas associated with both anal stenosis and anal ulcer (n = 2; 7%).
5. Primary and Secondary Outcomes: Complete and Partial Fistula Healing
All studies reported the primary outcome, rate of complete healing of perianal fistulizing disease (Fig. 2). In the randomized trial by Schwartz et al. [12], of the 28 initially enrolled patients with perianal disease, 8 had incomplete data at the time of primary outcome assessment, and those were counted as nonresponders in an intention-to-treat analysis.
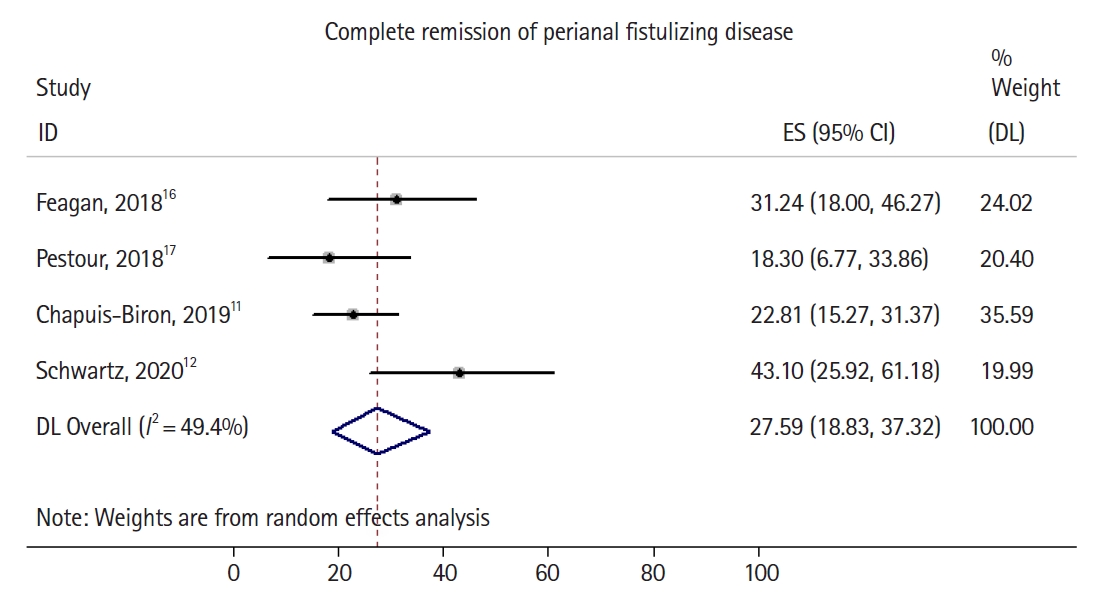
Forrest plot demonstrating pooled rate and 95% confidence interval (CI) of complete healing of perianal fistulizing disease. ES, effect size; DL, DerSimonian-Laird method.
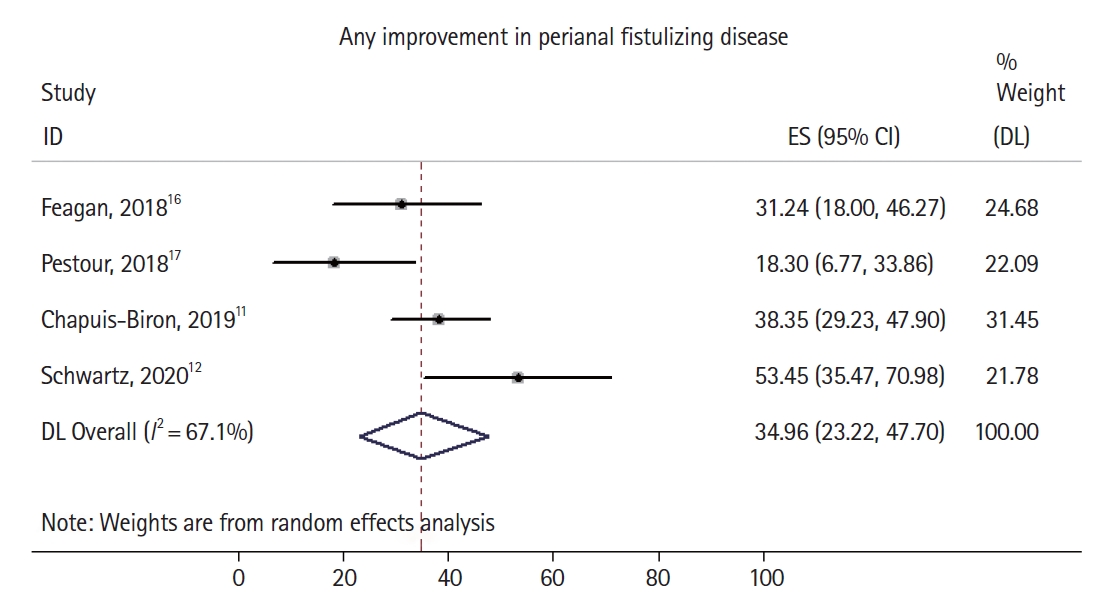
Overall, 52 out of 198 patients had complete healing of perianal fistulizing disease, with a pooled complete healing rate of 27.6% (95% confidence interval [CI], 18.9%–37.3%) and moderate heterogeneity identified in the pooled analysis (I2 = 49.4%). Two of 4 studies [11,12] explicitly reported the number of patients with partial healing (rather than complete healing) of perianal fistulizing disease (Fig. 3), with the 2 remaining studies only reporting rates of complete healing. A total of 71 out of 198 patients had at least partial healing of their perianal fistulizing disease, with a pooled partial healing rate of 34.9% (95% CI, 23.2%–47.7%) and high heterogeneity identified in the pooled analysis (I2 = 67.1%). The noted moderate to significant heterogeneity in both pooled estimates can likely be explained by differences in sample size, in the rates of previous anti-TNF failure, rates of concomitant steroid or immunomodulator use, lack of detailed information on seton use in all studies, and the variable severity of perianal disease in the studied cohorts. Sufficient patient data were not available to perform an analysis focused on patients who had failed anti-TNF compared to those who were biologic-naïve. There was no evidence of substantial publication bias based on visual inspection of the funnel plot (Supplementary Fig. 1).
6. Recurrence (or De Novo Incidence) of Perianal Disease in Patients with Inactive (or No) Disease at Baseline
Two studies [11,16] reported recurrence or de novo incidence of perianal disease in patients with inactive or no perianal disease at baseline. The randomized, controlled study by Schwartz only enrolled patients with active perianal disease and this outcome was not applicable. In a total of 357 patients, 23 (6%) had either de novo or recurrent perianal fistulizing disease during induction and maintenance treatment with vedolizumab. In the retrospective study by Chapuis-Biron et al., 15 out of 49 patients with inactive perianal disease had a recurrence and in the randomized trial by Feagan et al., 8 patients had de novo perianal disease while on vedolizumab.
7. Quality Assessment
The risk of bias in the 4 studies was evaluated according to the modified Newcastle-Ottawa assessment scale and is shown in Supplementary Table 2. Overall, 2 of the 4 studies were found to be of high quality, 1 moderate quality, and 1 was found to be low quality.
DISCUSSION
In our meta-analysis of 198 patients with perianal fistulizing CD, induction and maintenance therapy with vedolizumab led to complete healing of perianal fistulizing disease in 27.6% of patients and at least partial healing in 34.9%. Notably, the majority (87%) of included patients had failed prior anti-TNF therapy suggesting an already challenging disease phenotype. Nonetheless, our findings suggest vedolizumab may have numerically comparable perianal fistula healing rates to anti-TNF agents [20-22].
Our meta-analysis adds to the limited literature available for clinicians faced with management of refractory perianal fistulizing CD. The majority of included subjects in our analysis had failed previous anti-TNF therapy. The causes of failure (primary vs. secondary nonresponse, adverse events) were not adequately described in the included studies. Two of our studies were from France, where vedolizumab is only reimbursed after anti-TNF failure [11], explaining the predominance of patients who had failed anti-TNF therapy in those studies. In 1 included study [11], at least 81% of patients had failed 2 or more anti-TNF agents. Overall, these factors may suggest a more resistant disease phenotype in our analyzed cohort; such that vedolizumab may possibly have an even higher success rate in biologic-naïve patients as has been previously demonstrated in other studies of vedolizumab [23]. It is important to interpret our pooled results while considering the heterogeneity of the underlying study subjects. Several factors are known to modulate fistula healing including concurrent seton use, antibiotics, steroids and/or immunomodulators. Granular data were not available to perform subgroup analyses factoring in these different variables. For example, the study by Chapuis-Biron et al. [11] indicated antibiotic use was associated with a lower rate fistula healing, perhaps due to a more severe phenotype in that subgroup of patients.
In practice, vedolizumab is mostly used as second- or third-line therapy in fistulizing CD due to both payor issues and the lack of robust effectiveness data as compared to anti-TNF agents [24]. An examination of the mechanism of action for vedolizumab and TNF inhibitors, as well as the pathophysiology of fistulizing disease offers insights into their differential effectiveness in fistulizing disease. Vedolizumab is a monoclonal antibody against integrin α4β7 which is expressed on a subset of T-cells. In vivo, vedolizumab blocks the interaction between the α4β7 integrin and mucosal addressin cell adhesion molecule-1, which is mainly expressed on gastrointestinal tract endothelial cells [25]. This mechanism of action allows vedolizumab to be “gut-selective,” primarily reducing migration of T-lymphocytes into the gastrointestinal tract. In comparison, TNF inhibitors neutralize the activity of TNF-α in both its soluble and transmembrane forms across various tissues with downstream effects on several inflammatory pathways [26].
While inflammation may allow the initial epithelial defect for required for fistula formation, epithelial to mesenchymal transition (EMT) of intestinal epithelial cells leads to downregulation of cell adhesion molecules, such as epithelial cadherin, loss of epithelial cell polarity, and migration of mesenchymal-like cells into the deeper layers to form fistula tracts [27,28]. Multiple cytokines coordinate to promote the EMT to allow for deeper penetration of the migrating cells: TNF-α produced by inflammatory cells; transforming growth factor β and interleukin-13 produced by fibroblasts; and matrix metalloproteinases that degrade the extracellular matrix [29]. There is marked upregulation of TNF and the TNF receptor on transitional (mesenchymal-like) cells within perianal fistula tracts [30]. Given that TNF-α is a central regulator of both the inflammation that results in an initial epithelial defect and the EMT, it is not surprising that TNF neutralizing antibodies are effective in treating penetrating CD. The potential mechanism of more selective therapies, such as vedolizumab, in perianal CD is more recently being elucidated. A recent study of 7 patients with perianal fistulae found a significant number of T-cells in curettage material from the fistula tracts, and of the total CD3+ T-cells, 68.7% were CD3+ α4β7+. While not establishing a causal relationship, the presence of α4β7+ T-cells within fistula tracts lends some mechanistic support to the exploration of vedolizumab as a therapy for perianal fistulizing CD [31].
Our analysis has several limitations, largely owing to the quality and scarcity of data on vedolizumab in perianal fistulizing CD. The limited number of included studies and the underlying heterogeneity of study subjects may limit the generalizability of our results to the broader fistulizing CD population, particularly those with enteric and other non-perianal fistulas. While 2 of the included 4 studies were prospective randomized clinical trials, only 1 was a placebo-controlled trial but only for the maintenance phase of therapy, limiting our ability to generate odds ratios for fistula healing as compared to placebo. While direct comparisons to other therapies cannot be made due to different underlying patient populations, the numerical rates of response found in our study were comparable to those seen with anti-TNF agents [22], in an arguably more challenging disease phenotype since the majority of patients in our analysis had failed anti-TNF agents. To highlight these similarities, we extracted data on anti-TNF therapy for induction of healing of fistulizing CD as reported in a robust meta-analysis by Lee et al. [22] from a total of 6 controlled studies [4,20,32-35]. We used a similar statistical approach and found that anti-TNF therapy led to induction of complete healing of fistulizing CD in a total of 92 out of 267 patients, with a pooled rate of 29.7% (95% CI, 18.3%–42.6%). Finally, there was some variability in the time points and the modalities used for assessment of the primary outcome of fistula healing, widening the true CI of our pooled estimates.
A future randomized, placebo-controlled trial including biologic naïve patients with balanced underlying baseline characteristics and concurrent medical and surgical treatment characteristics would allow insights into the true efficacy of vedolizumab for fistulizing disease. However, the realities of CD clinical trial logistics and enrollment make the likelihood of such a trial being conducted quite low. This is exemplified by the difficulties faced by Schwartz et al. [12] in their well-designed international prospective, randomized, controlled trial for perianal fistulizing CD comparing standard vedolizumab dosing versus standard dosing plus an additional dose at week 10 (ENTERPRISE) which closed enrollment early after enrolling 34 subjects, only 20 of which ultimately had complete data for the final analysis. These limitations are increasingly recognized by the wider IBD research community, with a recent analysis finding that the average recruitment rate in moderate-to-severe CD decreased from 0.65 to 0.10 patients per site per month in the 20 years from 1998 to 2018 [36].
The management of perianal fistulizing CD continues to be challenging for both patients and physicians. While anti-TNF agents have demonstrated efficacy in induction of remission, long-term healing is achieved only in 50% of patients [37]. In this meta-analysis of 4 studies including 198 patients with perianal fistulizing CD, the majority of which had failed previous anti-TNF therapy, vedolizumab led to complete healing of perianal fistulizing disease in 27.6% of patients and at least partial healing in 34.9%. Future studies designed to control for concurrent surgical and medical therapies and enrolling a larger number of biologic naïve patients are warranted.
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Table 1. Search Strategy, Search from Inception through June 2020
ir-2021-00091-suppl1.pdfSupplementary Table 2. Modified Newcastle-Ottawa Quality Assessment Scale for Included Studies
ir-2021-00091-suppl2.pdfSupplementary Fig. 1. Funnel plot for included studies reporting complete healing of perianal fistulizing disease.
ir-2021-00091-suppl3.pdfNotes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
This was an investigator-initiated study. There was no involvement of the drug manufacturer in either the conception, data collection, analysis, or manuscript writing. Rubin DT is a consultant to AbbVie, AbGenomics, Allergan Inc., Amgen, Celgene Corporation, Forward Pharma, Genentech/Roche, Janssen Pharmaceuticals, Merck & Co Inc., Miraca Life Sciences, Mitsubishi Tanabe Pharma Development America Inc., Napo Pharmaceuticals, Shire, Takeda, and Target Pharma Solutions and has received grant support from AbbVie, Genentech/Roche, Janssen Pharmaceuticals, Prometheus Laboratories, Shire, Takeda, and UCB Pharma. Cohen RD serves on the speaker’s bureau for AbbVie and Takeda; serves as a consultant for AbbVie, Celgene, Janssen, Pfizer, and Takeda; and has conducted clinical trials for AbbVie, Boehringer Ingelheim, Celgene Corp., Crohn’s & Colitis Foundation of America, Genentech, Gilead, Hollister, Medimmune, Pfizer, Receptos, Schwarz Pharma, Seres Therapeutics, and Takeda. His spouse serves on the Board of Directors for Aerpio Therapeutics, Novus Therapeutics, and Vital Therapies, Inc. Pekow J is a consultant for Versatem and CVS Caremark and has received grant support from AbbVie and Takeda. He has served on the advisory board for Takeda, Pfizer and Jansen. Dalal SR is on the speaker’s bureau of AbbVie and has served as a consultant for Pfizer. The other authors declare no conflict of interest related to this work.
Rubin DT is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author Contribution
Guarantor of article: Ayoub F. Conceptualization: Ayoub F, Odenwald M, Micic D, Dalal SR, Pekow J, Cohen RD, Rubin DT, Sakuraba A. Data curation: Ayoub F, Odenwald M. Formal analysis: Ayoub F. Investigation: Odenwald M, Dalal SR, Pekow J, Cohen RD, Rubin DT, Sakuraba A. Methodology: Ayoub F, Odenwald M. Project administration: Ayoub F. Software: Ayoub F. Supervision: Micic D, Dalal SR, Pekow J, Cohen RD, Rubin DT, Sakuraba A. Writing - original draft: Ayoub F. Writing - review & editing: Ayoub F, Odenwald M, Micic D. Approval of final manuscript: all authors.