Korean clinical practice guidelines on biologics for moderate to severe Crohn’s disease
Article information
Abstract
Crohn’s disease (CD) is a relapsing and progressive condition characterized by diarrhea, abdominal pain, weight loss, and hematochezia that results in serious complications such as perforations, fistulas, and abscesses. Various medications, interventions, and surgical treatments have been used to treat CD. The Korean guidelines for CD management were distributed in 2012 and revised in 2017 by the Inflammatory Bowel Disease (IBD) Research Group of the Korean Association for the Study of Intestinal Diseases. Substantial progress in mucosal immunologic research has elucidated the pathophysiology of IBD, leading to development of biological agents for treatment of CD. The first developed biologic agent, tumor necrosis factor-α agents, were shown to be efficacious in CD, heralding a new era in management of CD. Subsequently, vedolizumab, a monoclonal antibody against integrin α4β7, and ustekinumab, a human monoclonal antibody that inhibits the common p40 subunit of interleukin-12 and interleukin-23, were both approved for clinical use and are efficacious and safe for both induction and maintenance of remission in moderate-to-severe CD patients. Moreover, a recent study showed the non-inferiority of CT-P13, an infliximab biosimilar, compared with infliximab in CD patients. The third Korean guidelines for CD management provide updated information regarding treatment of moderate-to-severe CD patients with biologic agents.
INTRODUCTION
Crohn’s disease (CD) is a chronic inflammatory intestinal disease of the digestive tract characterized by severe diarrhea, abdominal pain, weight loss, fatigue, and hematochezia [1,2]. It has a relapsing course, resulting in severe complications such as multiple bowel strictures, perforations, and abscesses in the gastrointestinal tract [2,3]. Nearly half of patients with CD undergo bowel surgery due to complications in their lifetime [4]. The incidence of CD has rapidly increased in Korea over the last two decades and has recently plateaued according to the inflammatory bowel disease (IBD) fact sheet in Korea [5]. However, the rapid increase in prevalence of CD is possibly attributable to the low mortality rate of patients [5]. Moreover, costs related to CD management have increased by 20% annually over the last 10 years, suggesting that CD is becoming an important economic burden in Korea [6].
Up-to-date management options for CD focus on inhibition or regulation of mucosal inflammation based on the mucosal immune pathway [7]. Suppression of tumor necrosis factor (TNF)-α, a dominant inflammatory cytokine in IBD, is associated with effective disease control [8]. Although the success of TNF-α receptor antagonists is a milestone in the management of CD, it remains challenging to treat primary non-responders [9]. In addition, 20% of cases may lose their response each year [10]. Therefore, new agents that target other immune pathways associated with the pathogenesis of CD must be developed. Substantial progress in mucosal immunologic research has elucidated the pathophysiology of IBD leading to the development of novel agents such as vedolizumab and ustekinumab [11,12].
Clinical management guidelines for CD were published in 2012 by the members of the IBD Research Group of the Korean Association for the Study of Intestinal Diseases (KASID) [13]. The guidelines provide approaches to deal with various issues associated with CD and to minimize disparities in management plans among physicians [13]. The second Korean guidelines were published in 2017 [14]. Recently, biologic agents including antiTNF-α, anti-integrin α4β7, and anti-interleukin (IL)-12/23 monoclonal antibodies and biosimilars have been used in real-world settings in Korea [15]. Therefore, the third Korean guidelines for management of CD need to be developed. These third guidelines focus on biologic therapies, including biosimilars, and management of CD, including management of perianal fistulas.
METHODS
1. Planning and Directions
The IBD research group of the KASID decided to develop the third guidelines for the management of CD in October 2020, to update the previous guidelines published in 2017. To establish the guidelines, the KASID selected a panel of 9 IBD experts (Seong-Joon Koh, Sung Noh Hong, Byong Duk Ye, Jeong Eun Shin, Kyeong Ok Kim, Hong Sub Lee, Soo-Kyung Park, Sung Hoon Jung, and Chang Hwan Choi) and 1 IBD surgeon (Yong Sik Yoon) supported by a methodologist (Miyoung Choi). The first meeting was held on October 5, 2020. Several novel biologic agents and biosimilars have been approved for management of moderate to severe CD in Korea. Diverse classes of biologics with different efficacies and safety profiles are available and therefore the third Korean guidelines focus on management of CD with biologic agents.
2. Process of Development
1) Selection of the Key Clinical Questions
The key questions were gathered and selected from those raised in the clinic by the board members. All questions were solved using adaptations. Each question regarding management of CD identified the population (P), intervention (I), comparison (C), and patient-important outcomes (O). The committee members performed a systematic search and review to assess the relevant data in order to address the clinical questions.
2) Assessment of Guideline Qualities and Final Selection
The committee members selected 516 articles published between January 1, 2015, and October 1, 2020, by searching MEDLINE, Embase, the Guidelines International Network, and Korean Medical Guideline Information Center using keywords including “Crohn,” “Crohn’s disease,” and “CD.” The selected guidelines were assessed by 2 independent committee members using Appraisal of Guidelines for Research & Evaluation II (AGREE II). Finally, we identified 6 guidelines that were evidence-based, peer-reviewed, and nationally or internationally appraised (Table 1) [14,16-20].
3) Development of Recommendations and Evidence Review
Recommendations from the 6 guidelines selected by AGREE II from 2015 to 2020 were reviewed to formulate a statement for the 18 clinical questions. Recent studies published after the 6 reference guidelines were identified via a PubMed search and included as evidence for our guidelines. References for each PICO question were evaluated for quality by 2 independent members of the CD guideline committee using RoB 1.0 (randomized controlled trials, RCTs) and RoBANS 2.0 (observational studies) and then presented in the technical review. However, the guideline committee for CD made no recommendation for some clinical questions because of the knowledge gap.
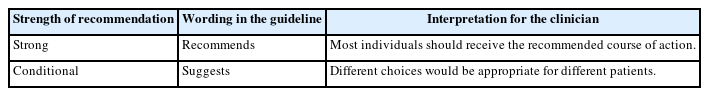
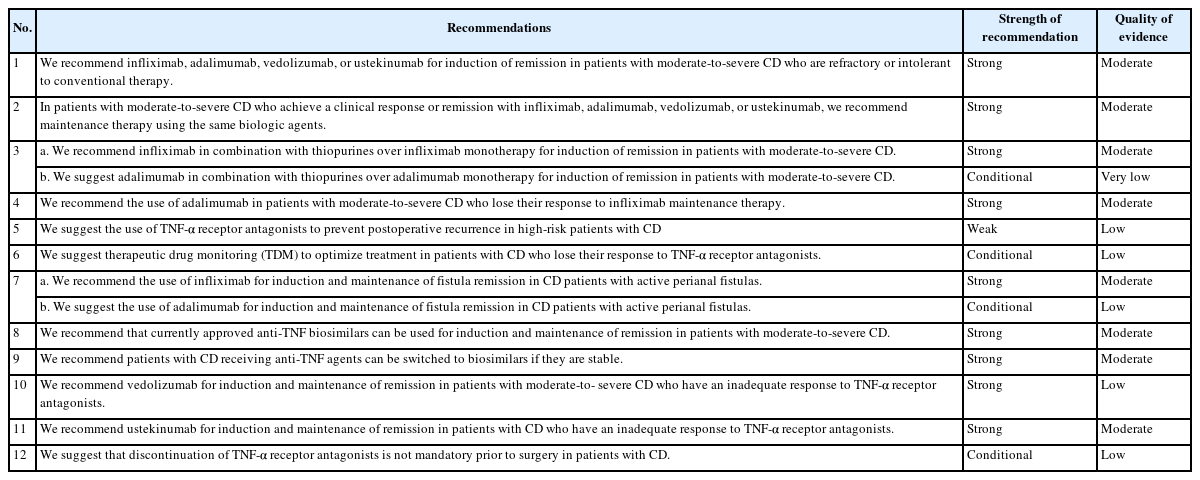
The evidence was assessed for quality and classified into 4 categories, from high to very low, based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) classification (Table 2) [21]. Each recommendation was carefully classified as “strong” or “conditional,” considering the quality of the evidence, values or preferences, and resource use (Table 3). The recommendations, certainty of evidence, and strength of recommendations are summarized in Table 4.
4) External Review, Endorsement, and Distribution of Guidelines
The draft was peer-reviewed by IBD experts among members of the KASID. All comments were collected, reviewed, and addressed by the guidelines committee.
3. Management of CD Using Biologic Agents
The goal of CD treatment is to prevent complications such as abscesses, strictures, and perforations by inducing and maintaining remission. The “treat-to-target” clinical management strategy has been used for management of IBD [22]. The Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) therapeutic goals for treat-to-target strategies were recently updated in STRIDE II [23]. Symptomatic improvements have been proposed as short-term therapeutic targets. Symptomatic and biochemical normalization, including calprotectin levels, have been suggested as intermediate targets. Endoscopic healing, normalization of quality of life, and absence of disability have been identified as long-term targets. Transmural healing in CD was not defined as a formal target but should be evaluated for a deep remission status [23].
Conventional treatment with steroids and immunomodulators cannot alter long-term prognosis. However, suppression of TNF-α in IBD is effective for mucosal healing, which reduces the risk of bowel surgery and recurrence after operations [24]. Recently, substantial progress in understanding of the immunopathogenesis of CD has been achieved through mucosal immunologic research, which has led to the development of novel therapeutic drugs for CD [7]. Vedolizumab, a monoclonal antibody against integrin α4β7, has shown efficacy and safety for induction of remission and was found to maintain remission in large clinical trials (GEMINI I and GEMINI II) of both CD and ulcerative colitis (UC) [25,26]. Importantly, no case of progressive multifocal leukoencephalopathy has been reported. This led to approval of vedolizumab for clinical use in 2014. Ustekinumab is a fully human monoclonal antibody that blocks the p40 subunit of IL-12 and IL-23, resulting in inhibition of the Th1 and Th17 pathways. Ustekinumab has been approved for clinical use and is effective and safe for inducing and maintaining remission in patients with moderate to severe CD and UC [27]. These novel agents have increased our ability to achieve the goals of CD treatment.
Biologic agents including anti-TNF biosimilars are available in real-world practice in Korea. Unfortunately, little information is available regarding head-to-head clinical trials comparing the efficacy and safety of biologics for patients with CD. Although network meta-analyses have been performed, limited information exists regarding the choice of biological agents for CD [28].
4. Definition of CD
1) Disease Location and Behavior
The Vienna Classification has been previously used to classify CD [29]. The Montreal classification was proposed in 2005 (Table 5) [30]. The CD guideline committee agreed that CD should be classified according to the Montreal classification because this is useful for describing phenotypes. Age of onset ( ≤ 16, 17–40, > 40 years), disease location (ileum, colon, ileocolon, and upper gastrointestinal), and disease behavior (non-stricturing/non-penetrating, stricturing, penetrating, and perianal disease modifier) were considered.
2) Clinical Disease Activity
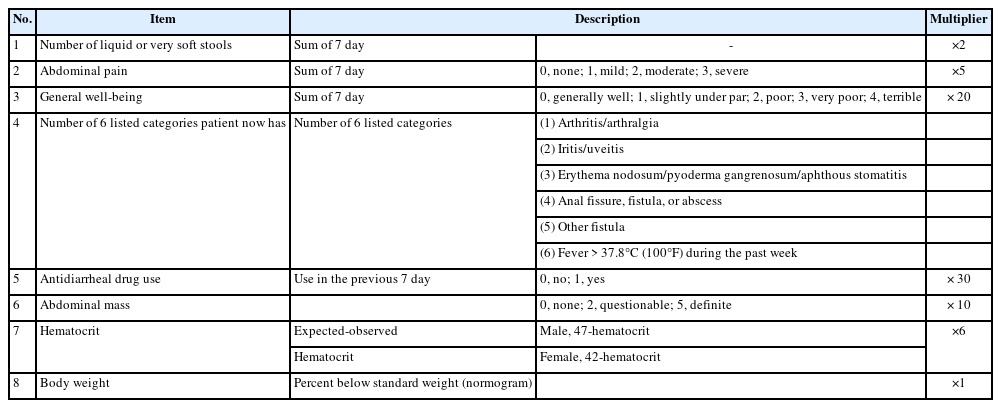
The Crohn’s Disease Activity Index (CDAI) has been widely used in clinical trials and practice (Table 6) [31]. A CDAI < 150, 150–220, 220–450, ≥ 450 is classified as clinical remission, mild clinical activity, moderate clinical activity, and severe clinical activity, respectively. This system is limited because it has not been validated in patients who have undergone surgery.
3) Definition of Terminologies
Clinical response is defined as a decrease in score to CDAI ≥ 100 (CDAI-100) [18]. In some clinical trials, a decrease in the CDAI to at least 70 (CDAI-70) is determined to be a clinical response [32,33]. The Harvey-Bradshaw index (HBI) defines it as a decrease of ≥ 3 in the scoring system [34]. Relapse is defined as the recurrence of symptoms with a CDAI ≥ 150 in patients in clinical remission [31]. In the HBI scoring system, relapse is defined as a HBI > 4 [35].
Steroid-refractory CD patients are those in whom remission is not induced or who do not respond to an adequate treatment dosage of 0.75 mg/kg/day prednisolone within 4 weeks. Steroid-dependent CD patients are those who respond to steroid therapy but in whom the dose of prednisolone cannot be reduced to < 10 mg/day or who suffer symptom recurrence within 3 months after steroid discontinuation [36].
Statement 1
We recommend infliximab, adalimumab, vedolizumab, or ustekinumab for induction of remission in patients with moderate-to-severe CD who are refractory or intolerant to conventional therapy. (Strong recommendation with a moderate level of evidence)
RCTs demonstrated that biologics such as infliximab, adalimumab, vedolizumab, and ustekinumab are more efficacious than placebo for inducing remission in patients with luminal CD who have moderate to severe activity. Infliximab was more efficacious than the placebo for achieving clinical remission in 2 RCTs involving 106 biologic-naïve patients with CD [8,37]. In 1 trial, the clinical remission rate at week 4 was significantly higher in patients who received a single injection of different doses of infliximab than in those who received the placebo [8]. In an RCT performed by GETAID, the steroid-free clinical remission rate at week 24 was higher in the infliximab-treated group than in the placebo-treated group (57% vs. 29%, P= 0.003) [37]. In 3 RCTs including 531 patients with CD, adalimumab was significantly more efficacious than the placebo for achieving clinical remission [38,39]. The CLASSIC-1 trial was conducted of patients who had never received biologics, whereas the GAIN trial exclusively included CD patients who had prior intolerance or secondary loss of response to infliximab. Three RCTs involving 969 patients to evaluate the efficacy of vedolizumab reported that vedolizumab was effective for inducing clinical remission within 6–10 weeks, compared with the placebo (relative risk [RR], 2.01; 95% confidence interval [CI], 1.50–2.71) [25,40,41]. In these RCTs, 50% to 75% of patients were previously exposed to TNF-α receptor antagonists [25,40,41]. In 3 RCTs, ustekinumab induced a higher rate of clinical remission than placebo in patients with moderately-to-severely active CD (RR, 1.76; 95% CI, 1.40–2.22) [27,42]. Two trials only included patients who had been previously exposed to TNF-α receptor antagonists [27,42]. No head-to-head comparative trial has been conducted of biologics in patients with moderate-to-severe CD. Network meta-analyses compared different agents. However, the results should be treated with some caution due to heterogeneity in the study design and characteristics of patients enrolled. Physicians should consider the patient’s preference, cost, likely adherence, safety, and speed of response to the drug when choosing biologics [17]. Although biologic therapy can be a meaningful treatment option for patients with CD, its impact on clinical response and remission is lower in patients who have been previously exposed to biologic therapies [43].
Statement 2
In patients with moderate-to-severe CD who achieve a clinical response or remission with infliximab, adalimumab, vedolizumab, or ustekinumab, we recommend maintenance therapy using the same biologic agents. (Strong recommendation with a moderate level of evidence)
Nine RCTs compared the efficacy for maintenance of remission in patients who previously had moderately-to-severely active CD after induction of remission with biologics including infliximab, adalimumab, vedolizumab, and ustekinumab. In the ACCENT-1 trial in which 335 patients with moderately to severely active CD responded to a single infusion of infliximab within 2 weeks, infliximab was more efficacious than placebo to maintain clinical remission at week 30 (odds ratio [OR], 2.7; 95% CI, 1.6–4.6) and at week 54 (OR, 4.2; 95% CI, 1.5–11.5) [33]. In 3 RCTs in which 422 patients with moderatelyto-severely active CD who responded to standard induction treatment were re-randomized to receive adalimumab or placebo, adalimumab was more efficacious than the placebo to maintain clinical remission [44-46]. In the GEMINI-2 trial, in which 461 patients with CD who achieved clinical remission or a response to induction therapy at week 6 or 10 were re-randomized to receive vedolizumab or placebo, vedolizumab was significantly more efficacious to maintain clinical remission at week 46 (RR, 1.81; 95% CI, 1.26–2.59) [25]. In the CERTIFI and UNITI trials, which included patients who achieved a clinical response to ustekinumab at week 6 or 8, maintenance treatment with ustekinumab every 8 weeks (53.1%) or 12 weeks (48.8%) resulted in a higher rate of clinical remission at week 44 than treatment with placebo (35.9%; P= 0.005 and P= 0.04, respectively) [27].
Statement 3
a. We recommend infliximab in combination with thiopurines over infliximab monotherapy for induction of remission in patients with moderate-to-severe CD. (Strong recommendation with a moderate level of evidence)
b. We suggest adalimumab in combination with thiopurines over adalimumab monotherapy for induction of remission in patients with moderate-to-severe CD. (Conditional recommendation with a very low level of evidence)
One RCT (SONIC study) evaluated the efficacy of infliximab plus a thiopurine in patients with moderately-to-severely active CD [47]. Of the 169 patients who received both infliximab and azathioprine, 96 (56.8%) achieved corticosteroid-free clinical remission at week 26, while 75 patients (44.4%) who received infliximab monotherapy achieved steroid-free clinical remission (P= 0.02). Comparable tendencies were observed at week 50. At week 26, 47 of 107 patients (43.9%) receiving combination therapy achieved mucosal healing. However, only 28 of 93 patients (30.1%) achieved mucosal healing in the infliximab monotherapy group (P= 0.06). No RCT has compared maintenance therapy with infliximab plus a thiopurine and infliximab alone in CD patients. However, the SONIC trial additionally showed that efficacy of a combination regimen to maintain remission with a subgroup of CD patients who entered a blinded extension for 50 weeks. Of the 108 patients treated with infliximab plus azathioprine, 28 patients failed to maintain corticosteroid-free clinical remission, compared with 33 out of 97 infliximab monotherapy–treated patients (RR, 0.76; 95% CI, 0.50–1.16).
The DIAMOND trial evaluated the efficacy of adalimumab plus azathioprine versus adalimumab monotherapy in patients with moderately-to-severely active CD over 52 weeks [48]. At 26 weeks, failure to achieve clinical remission did not significantly differ between the 2 groups (28/91 vs. 20/85: RR, 1.31; 95% CI, 0.80–2.14). However, combination therapy was associated with a significantly higher rate of endoscopic remission than adalimumab monotherapy at week 26 (48/57 [84.2%] vs. 37/58 [63.2%], P= 0.02). Upon extension to 52 weeks, maintenance of clinical remission did not significantly differ between the combination treatment group and adalimumab single regimen (29/91 vs. 24/85: RR, 1.13; 95% CI, 0.72–1.78). The proportion of patients with endoscopic remission did not significantly differ between the 2 groups both at randomization and week 52 (39/49 [79.6%] vs. 37/53 [69.8%], P= 0.36). The Personalized Anti-TNF Therapy in Crohn’s Disease Study (PANTS) enrolled anti-TNF-naive patients with active luminal CD at the time of first exposure to infliximab or adalimumab and evaluated them for 12 months. Combination immunomodulator (thiopurine or methotrexate) therapy mitigated the risk of developing anti-drug antibodies not only for infliximab (hazard ratio, 0.39; 95% CI, 0.32–0.46, P< 0.0001) but also for adalimumab (hazard ratio, 0.44; 95% CI, 0.31–0.64, P< 0.0001) [49].
A double-blind RCT evaluated the efficacy of infliximab plus methotrexate and infliximab monotherapy among 126 patients with CD [50]. Failure to induce corticosteroid-free clinical remission at weeks 14 and 50 did not significantly differ between the 2 groups. However, higher infliximab trough concentrations indirectly suggested that immunogenicity was lower in patients who received combination therapy than in patients who received the infliximab single regimen.
Statement 4
We recommend the use of adalimumab in patients with moderate-to-severe CD who lose their response to infliximab maintenance therapy. (Strong recommendation with a moderate level of evidence)
Treatment failure with TNF-α receptor antagonists includes primary treatment failure and secondary loss of response. Development of immunogenicity is critical for secondary loss of response. There is no evidence of an anti-drug antibody crossreacting with other TNF inhibitors. Based on a systematic review and meta-analysis of the efficacy of a second TNF-α receptor antagonist in patients with IBD in whom TNF-α receptor antagonist treatment has failed, the efficacy of a second TNF-α receptor antagonist in CD patients largely depends on the reason for switching [51]. The remission rate is higher when the reason to withdraw the first TNF-α receptor antagonist is intolerance (61%) than what it is secondary (45%) or primary (30%) failure.
The GAIN trial demonstrated the efficacy of adalimumab among patients with moderate-to-severe CD with secondary infliximab failures including intolerance [39]. An open-label extension trial was performed to evaluate the efficacy of adalimumab for maintaining remission among CD patients who completed the GAIN trial [52]. The percentage of patients with CDAI-70, CDAI-100, and clinical remission at week 96 were 39.0%, 35.5%, and 26.5%, respectively. Moreover, adalimumab was effective for achieving remission among Japanese patients with CD who had been exposed to TNF-α receptor antagonists compared with the placebo (5/19 vs. 1/13) [46].
However, no clinical trial has investigated the effect of infliximab after loss of response to adalimumab. In a large cohort study, the remission rate did not significantly differ between patients who received adalimumab followed by infliximab and those who received infliximab followed by adalimumab (48% vs. 42%, P= 0.07) [53]. In another study, an adalimumab and infliximab treatment sequence maintained a response in 78% of patients at week 54 [54]. Therefore, infliximab should be carefully considered as a second-line agent in patients with CD who lose response to adalimumab.
Statement 5
We suggest the use of TNF-α receptor antagonists to prevent postoperative recurrence in high-risk patients with CD. (Weak recommendation with a low level of evidence)
Symptomatic recurrence is observed in approximately 40% of patients with CD within 5 years after bowel surgery. In addition, endoscopic recurrence in a postoperative setting is frequently detected in up to 97% of patients with CD within 1 year [55]. During long-term follow-up over 88 months, 48% of patients with CD exhibited recurrence after surgery [56]. A meta-analysis reported that endoscopic recurrence was observed at 52 weeks in 58% of patients who underwent bowel surgery [57]. Smoking, multiple bowel surgeries, and penetrating disease are risk factors for recurrence after surgery [58,59]. In addition, patients with concomitant small and large bowel involvement, immunomodulator use, residual active disease, and preoperative disease activity were at higher risk of postoperative recurrence [60,61].
Several RCTs have reported the efficacy of TNF-α receptor antagonists to reduce postoperative recurrence in patients with CD. The recurrence rate was lower among patients treated with infliximab (1/11 patients, 9.1%) than among those treated with the placebo (11/13 patients, 84.6%) (P= 0.0006) [62]. Another prospective, randomized, single-center, open trial with 2 arms showed that 100% and 93.3% of patients administered infliximab maintained remission at 12 and 36 months, respectively, compared with 68.8% and 56.3% of patients in the control group, respectively (P< 0.03) [63]. A large, international, multicenter RCT with 297 participants demonstrated that infliximab significantly reduced endoscopic recurrence compared with the placebo (30.6% vs. 60.0%, P< 0.001) [64]. However, infliximab did not effectively reduce clinical recurrence (P= 0.097).
The effect of adalimumab on recurrence after surgery was evaluated in a randomized, 3-arm study. Adalimumab effectively reduced endoscopic (adalimumab, 6.3%; azathioprine, 65.7%; 5-ASA, 83.3%) and clinical (adalimumab, 12.5%; azathioprine, 64.7%; 5-ASA, 50%) recurrence within 2 years [65].
In a meta-analysis comprising 2,006 participants that compared 7 treatment strategies, mesalamine (RR, 0.60; 95% credible interval [CrI], 0.37–0.88), antibiotics (RR, 0.26; 95% CrI, 0.08–0.61), immunomodulator monotherapy (RR, 0.36; 95% CrI, 0.17–0.63), immunomodulator with antibiotics (RR, 0.11; 95% CrI, 0.02–0.51), and TNF-α receptor antagonist monotherapy (RR, 0.04; 95% CrI, 0.00–0.14), but not budesonide (RR, 0.93; 95% CrI, 0.40–1.84), reduced the risk of clinical relapse, compared with the placebo. Likewise, antibiotics (RR, 0.41; 95% CrI, 0.15–0.92), immunomodulator monotherapy (RR, 0.33; 95% CrI, 0.13–0.68), immunomodulator with antibiotics (RR, 0.16; 95% CrI, 0.04–0.48), and TNF-α receptor antagonist monotherapy (RR, 0.01; 95% CrI, 0.00–0.05), but not mesalamine (RR, 0.67; 95% CrI, 0.39–1.08) and budesonide (RR, 0.86; 95% CrI, 0.61–1.22), reduced the risk of endoscopic relapse [66]. TNF-α antagonist monotherapy was the most effective pharmacologic intervention for preventing postoperative recurrence, with large effect sizes relative to all the other strategies (clinical relapse: RR, 0.02–0.20; endoscopic relapse: RR, 0.005–0.04). Moreover, a recent meta-analysis including ten RCTs, incorporating 751 patients reported that TNF-α receptor antagonists showed efficacy for preventing endoscopic recurrence compared with the control (RR, 0.13; 95% CI, 0.04–0.39) [67].
Statement 6
We suggest therapeutic drug monitoring (TDM) to optimize treatment in patients with CD who lose their response to TNF-α receptor antagonists. (Conditional recommendation with a low level of evidence)
One RCT reported that TDM in patients who lose their response to anti-TNF therapy was associated with reduced treatment costs. However, there was no significant difference in clinical response rates, compared with routine dose intensification [68]. There are 3 observational studies evaluating the association between TDM and clinical outcomes in patients with IBD. Among IBD patients who lost their response to infliximab, half achieved mucosal healing after dose intensification. This response was associated with increased trough levels [69]. However, recent evidence reveals that primary nonresponders have lower drug levels than responders. In addition, significant antibody formation can occur within a few weeks of treatment initiation [70]. A recent study that analyzed 247 patients with 330 events of loss of response to TNF-α receptor antagonists (188 to infliximab and 142 to adalimumab) demonstrated that measurements of trough drug levels and anti-TNF-α receptor antagonist antibody formation could provide information regarding the likely outcome of interventions after loss of treatment response. Patients who had adequate trough levels (adalimumab > 4.5 μg/mL or infliximab > 3.8 μg/mL) or anti-TNF-α receptor antagonist antibodies (adalimumab > 4 μg/mL equivalent or infliximab > 9 μg/mL equivalent) improved more upon switching to an alternative TNF-α receptor antagonist or an alternative class of drug. However, dose intensification improved outcomes of patients with low trough levels and who were negative for anti-TNF-α receptor antagonist antibodies [71]. Taken together, reactive TDM does not guarantee better outcomes, but may have cost-saving effects.
Statement 7
a. We recommend the use of infliximab for induction and maintenance of fistula remission in CD patients with active perianal fistulas. (Strong recommendation with a moderate level of evidence)
b. We suggest the use of adalimumab for induction and maintenance of fistula remission in CD patients with active perianal fistulas. (Conditional recommendation with a low level of evidence)
Infliximab is the only TNF-α receptor antagonist whose efficacy in patients with fistulizing CD has been evaluated in 2 RCTs. Among 94 CD patients who had symptomatic draining fistulas, fistulas were completely closed more frequently within 18 weeks among those administered infliximab than among those administered the placebo (RR, 0.52; 95% CI, 0.34–0.78) [72]. In 1 RCT including 194 CD patients who had a fistula response after induction treatment (90% perianal), infliximab maintenance therapy was effective for fistula remission at 54 weeks [73].
No RCT has investigated the use of adalimumab for induction or maintenance of remission using fistula remission as the primary outcome. Adalimumab did not effectively induce complete fistula closure (RR, 1.08; 95% CI, 0.93–1.27) in subgroup analyses of 2 clinical trials of 77 patients with symptomatic fistulas [39,74]. However, a subgroup analysis showed that administration of adalimumab resulted in a better outcome in terms of complete fistula healing than the placebo (RR, 0.73; 95% CI, 0.54–0.97) [38].
Non-TNF-α receptor antagonists such as vedolizumab and ustekinumab can also be suggested over no treatment for fistulizing CD. However, evidence to support this is limited [75].
Statement 8
We recommend that currently approved anti-TNF biosimilars can be used for induction and maintenance of remission in patients with moderate-to-severe CD. (Strong recommendation with a moderate level of evidence)
A biosimilar is defined as a biotherapeutic product that is similar to a licensed reference biotherapeutic product in terms of quality, safety, and efficacy [76]. Because of their economic benefits, the introduction of biosimilars of biologic agents has been expected to reduce barriers for use of biologic agents in patients with IBD. CT-P13 is the first biosimilar of infliximab and has a similar pharmacokinetic profile as infliximab [77-81]. Clinically, the efficacy and safety of CT-P13 were first demonstrated in patients with rheumatologic arthritis (RA; PLANETRA study) [78,79] and ankylosing spondylitis (PLANETAS study) [80,81] Based on extrapolation of indications, CT-P13 was approved for all indications of infliximab [82]. However, there have been concerns about whether the efficacy and safety of CT-P13 proven in rheumatologic diseases can also be guaranteed in CD. Recently, a randomized, multicenter, double-blind, phase 3 non-inferiority study (PALETCD) compared the efficacy and safety of CT-P13 with those of infliximab in 220 CD patients [83]. TNF-α receptor antagonist-naïve adult patients with moderate-to-severe CD were randomly assigned (1:1) to the CT-P13 or infliximab group. At week 6, the CDAI-70 response rates were similar in the CT-P13 group (77/111, 69.4%; 95% CI, 59.9–77.8) and infliximab group (81/109, 74.3%; 95% CI, 65.1–82.2). The difference between the 2 CDAI-70 response rates was –4.9% (95% CI, –16.9% to 7.3%), fulfilling the non-inferiority criteria. The proportion of patients who achieved CDAI-100 and clinical remission (defined as an absolute CDAI < 150) at week 6 was also similar in the 2 groups as were the CDAI-70 response, CDAI-100 response, and clinical remission rates at weeks 14 and 30. Treatment-emergent adverse events were observed in 63 patients (56.8%) in the CT-P13 group and 70 patients (64.2%) in the infliximab group up to week 30. Development of treatment-emergent serious adverse events was also comparable between the 2 groups up to week 30 (5.4% in the CT-P13 group vs. 8.3% in the infliximab group).
In a study using the French nationwide health administrative database, 5,050 infliximab-naïve patients with CD had started treatment with infliximab (n = 2,551) or CT-P13 (n = 2,499), and a composite endpoint of death, CD-related surgery, all-cause hospitalization, and reimbursement of another biologic therapy was compared between the 2 groups as the primary outcome [82]. CT-P13 was equivalent to infliximab in terms of the primary outcome, and safety outcomes did not differ between the 2 groups.
The subcutaneous formulation of CT-P13 (CT-P13 SC) was recently developed and was compared with intravenous (IV) CT-P13 in a phase 1, randomized, double-blind, multinational, multicenter trial [84]. After IV CT-P13 injection at weeks 0 and 2 (5 mg/kg), active patients with CD or UC were randomized to continued IV CT-P13 injection arm (5 mg/kg every 8 weeks) or CT-P13 SC arm (120 mg [patients < 80 kg] or 240 mg [patients ≥ 80 kg] every 2 weeks). The primary endpoint, pharmacokinetic noninferiority of CT-P13 SC to CT-P13 IV at week 22, was met and the comparable efficacy, safety, and immunogenicity profiles were observed between the 2 groups up to week 30. CT-P13 IV group was switched to CT-P13 SC at week 30 and efficacy, safety, and immunogenicity were also broadly similar with CT-P13 SC maintenance group up to week 54.
Another biosimilar of infliximab, SB2, was compared with infliximab in patients with moderate-to-severe RA despite methotrexate therapy in a phase 3, randomized, double-blind, multinational, multicenter parallel group trial [85]. Up to week 30, SB2 was equivalent to infliximab in terms of the American College of Rheumatology 20% (ACR20) response [85]. The 2 drugs were also comparable in terms of other outcomes such as treatment-emergent adverse events, incidence of antidrug antibodies, and the pharmacokinetic profile [85]. The similarity of the 2 agents in terms of efficacy, safety and immunogenicity was maintained up to week 54 [86].
BI 695501, a biosimilar drug of innovator adalimumab has shown equivalent efficacy, highly similar safety and immunogenicity compared with the innovator in patients with RA (VOLTAIRE-RA) and in those with chronic plaque psoriasis (VOLTAIRE-PSO) through phase 3 randomized clinical trials [87,88]. In the phase 3 VOLTAIRE-CD randomized, double-blind trial, BI 695501 was compared with the innovator adalimumab for patients with moderate to severe CD [89]. At week 4 (after 160 mg at day 1 and 80 mg at day 15, via SC injection), a similar proportion of patients in the BI 695501 group showed a clinical response (CDAI-70) compared with the innovator adalimumab group (90% vs. 94%: adjusted risk ratio, 0.945; 90% CI, 0.870–1.028). Up to week 24, similar efficacy and safety were observed between the 2 groups. CT-P17 and SB5, another biosimilar of adalimumab, were compared with adalimumab in patients with moderate-to-severe RA in randomized, double-blind phase 3 trials. These 2 drugs were equivalent to adalimumab in terms of ACR20 response up to week 24 [90,91]. In addition, there was no difference between the biosimilars and adalimumab in terms of safety, immunogenicity, and pharmacokinetics [90,91].
Although no RCT has compared SB2, CT-P17, and SB5 with the innovator drugs in patients with CD, they will be equally efficacious and safe for patients with moderate-to-severe CD, based on extrapolation of indications [92].
Statement 9
We recommend patients with CD receiving anti-TNF agents can be switched to biosimilars if they are stable. (Strong recommendation with a moderate level of evidence)
The NOR-SWITCH trial was a 52-week, randomized, double-blind, phase 4 non-inferiority study that compared continuing treatment with infliximab and switching from infliximab to CT-P13 among stable patients with immune-mediated inflammatory diseases in terms of efficacy, safety, and immunogenicity [93]. Overall, disease worsening was observed in 53 out of 202 patients (26.2%) in the infliximab maintenance group and in 61 out of 206 patients (29.6%) in the CT-P13 switching group, with an adjusted treatment difference of –4.4% (95% CI, –12.7% to 3.9%), establishing the non-inferiority of switching. The proportions of patients experiencing adverse events (70% vs. 68%), serious adverse events (10% vs. 8%), and adverse events leading to study drug discontinuation (4% vs. 3%) were also similar in the 2 groups. The trough drug concentrations and frequencies of anti-drug antibodies were also similar in the 2 groups. In subgroup analysis of patients with CD, disease worsening was observed in 14 out of 66 patients (21.2%) in the infliximab maintenance group and in 23 out of 63 patients (36.5%) in the CT-P13 switching group [93,94]. The adjusted risk difference was –14.3% with a 95% CI of –29.3% to 0.7%, which was close to a significant difference in terms of favoring continuation of infliximab. However, at week 52, clinical remission was observed in 46 out of 66 patients (70%) in the infliximab maintenance group and in 41 out of 63 patients (65%) in the CT-P13 switching group (adjusted risk difference, 5.6%; 95% CI, –11% to 22.2%). In the subsequent extension study (for an additional 26 weeks) after the 52 weeks of the NOR-SWITCH trial, efficacy, safety, and immunogenicity in patients who received CT-P13 for 78 weeks (maintenance group) were compared with those in patients who switched to CT-P13 from infliximab at week 52 (switching group) [95]. At week 78, disease worsening was observed in 32 out of 190 patients (16.8%) in the maintenance group and in 20 out of 173 patients (11.6%) in the switching group. The adjusted risk difference was 5.9% (95% CI, –1.1% to 12.9%). The frequencies of adverse events and anti-drug antibodies were also comparable in the 2 groups. In analysis of the subgroup of patients with CD, 13 out of 63 patients (20.6%) in the maintenance group and 8 out of 61 patients (13.1%) in the switching group showed disease worsening. The 95% CI of the adjusted risk difference of 7.9% was –5.2% to 21%, which was not statistically significant. However, the NOR-SWITCH trial and its extension study were not powered to detect non-inferiority for individual diseases.
At week 54 of a phase 3, randomized, double-blind trial comparing SB2 and infliximab in patients with RA [85,86], patients receiving infliximab were re-randomized (1:1) to switch to SB2 or to continue receiving infliximab, whereas patients in the SB2 group continued to receive SB2 [96]. The efficacy, safety and immunogenicity profiles remained comparable among the 3 groups up to week 78 [96].
In the phase 3 VOLTAIRE-CD trial, the innovator adalimumab group was switched to BI 695501 at week 24 and efficacy up to week 48 and safety up to week 56 were comparable with the BI 695501 maintenance group [89].
At week 24 of a phase 3, randomized, double-blind trial comparing SB5 and adalimumab in patients with RA [91], patients receiving adalimumab were re-randomized (1:1) to continue with receiving adalimumab or to switch to SB5 up to week 52 in a transition study [97]. Patients in the SB5 group continued to receive SB5 for 52 weeks [97]. After switching, efficacy, safety, and immunogenicity were comparable across the treatment groups [97].
Based on the NOR-SWITCH study, its extension, and transition studies of RA patients, and considering the indication extrapolation, stable patients with CD can be switched to biosimilars without issues such as loss of efficacy, increased occurrence of adverse events, or increased immunogenicity [92].
Statement 10
We recommend vedolizumab for induction and maintenance of remission in patients with moderate-to-severe CD who have an inadequate response to TNF-α receptor antagonists. (Strong recommendation with a low level of evidence)
In the GEMINI 3 trial, vedolizumab induction treatment was not effective compared with the placebo in patients with moderately-to-severely active CD with an inadequate response to TNF-α receptor antagonists (clinical remission at week 6, 15.2% vs. 12.1%, P= 0.433). A higher proportion of patients administered vedolizumab had a CDAI-100 response at weeks 6 and 10 than those administered the placebo (39.2% vs. 22.3%, P= 0.001, RR of 1.8; 46.8% vs. 24.8%, P< 0.0001, RR of 1.9, respectively). The remission rate at week 10 was higher among patients administered vedolizumab than among those administered the placebo (26.6% vs. 12.1%, P= 0.001, RR of 2.2) [41]. In the GEMINI 2 trial, patients administered vedolizumab were more likely to achieve clinical remission than those administered the placebo (14.5% vs. 6.8%, P= 0.02), but were not more likely to have a CDAI-100 response. Among patients who responded to vedolizumab at week 6, the clinical remission rate at week 52 was significantly higher among those who received vedolizumab every 8 or 4 weeks than among those who received the placebo (P< 0.001 and P= 0.004, respectively) [25]. A recent Japanese study showed a numerically greater efficacy of vedolizumab for inducing and maintaining remission in patients with moderately-to-severely active CD and an inadequate response to TNF-α receptor antagonists, but the difference was not statistically significant [98]. In a meta-analysis of the real-world effectiveness of vedolizumab (80.4% of patients had previously been treated with TNF-α receptor antagonists), 30% of CD patients were in remission at week 14 (95% CI, 25%–34%) and month 12 (95% CI, 20%–45%). The rates of corticosteroid-free remission were 25% at week 14 (95% CI, 20%–31%) and 31% at month 12 (95% CI, 20%–45%) [99]. These findings suggest that vedolizumab effectively induces and maintains remission in patients with moderately-to-severely active CD and an inadequate response to TNF-α receptor antagonists.
Combination therapy with vedolizumab and a thiopurine was not effective compared with vedolizumab monotherapy in patients with CD. A recent meta-analysis reported that combination treatment with vedolizumab and an immunomodulator was not more effective than treatment with vedolizumab alone for 54 weeks [100]. Two recent guidelines suggest that the effectiveness of these combination therapies is unclear or that they are not recommended [20,101].
Statement 11
We recommend ustekinumab for induction and maintenance of remission in patients with CD who have an inadequate response to TNF-α receptor antagonists. (Strong recommendation with a moderate level of evidence)
Ustekinumab has been approved for clinical use and is efficacious to induce and maintain remission in patients with moderately-to-severely active CD (UNITI-1 and 2). UNITI-1 registered patients with CD and primary or secondary TNF-α receptor antagonist failure. Among CD patients who received 6 mg/kg or 130 mg ustekinumab, the clinical response was 37.8% and 33.5% at week 8, respectively. These rates were significantly higher than those in patients who received the placebo (20.2%) [27]. Growing real-world data confirmed the effectiveness of ustekinumab in CD. According to the Dutch Initiative on Crohn and Colitis, which enrolled 211 CD patients (98.6% of whom had been exposed to TNF-α receptor antagonist), the steroid-free remission rates at 24 and 52 weeks were 38.2% and 37.1%, respectively [102]. In addition, 54.4% of patients who received ustekinumab attained clinical remission among patients with CD in whom TNF-α receptor antagonist treatment had previously failed [103].
No clinical trial has addressed the efficacy of combination therapy with azathioprine and ustekinumab compared with ustekinumab monotherapy. Among patients enrolled in the UNITI trials, concomitant immunosuppressant use resulted in a numerically higher symptomatic response in CD patients. However, statistical assessment was not performed [27]. Two recent meta-analyses reported that the clinical response/remission (58.1% vs. 41.2%, P= 0.14) and endoscopic remission (58.1% vs. 41.2%, P= 0.14) rates at week 54 did not differ between patients who received combination therapy and those who received monotherapy [104,105]. By contrast, another meta-analysis reported that ustekinumab with immunosuppressants was more effective than ustekinumab without immunosuppressants as induction therapy (pooled OR, 1.35; 95% CI, 1.06–1.71; P= 0.015) [106]. Because of the lack of evidence and conflicting results of meta-analyses, the taskforce cannot make a specific recommendation about the use of combination therapy with azathioprine and ustekinumab over monotherapy with ustekinumab.
Some studies have shown that dose intensification of ustekinumab is effective in patients with CD who fail to respond to the standard dose. However, these results should be carefully interpreted because of the relatively small sample size and short follow-up periods [107-110].
Statement 12
We suggest that discontinuation of TNF-α receptor antagonists is not mandatory prior to surgery in patients with CD. (Conditional recommendation with a low level of evidence)
No RCT has assessed whether use of TNF-α receptor antagonists cause development of postoperative complications, which has been debated. Earlier meta-analyses of preoperative treatment with TNF-α receptor antagonists showed slight increases in postoperative complications including infectious adverse events (OR, 1.52; 95%; CI, 1.14-2.03; 8 studies) [111-116]. However, the most recent meta-analyses about this subject found no difference in the occurrence of any complications [117,118]. Current evidence suggests that cessation of TNF-α receptor antagonists prior to surgery is not mandatory because these drugs are deemed safe to use in the perioperative period.
CONCLUSIONS
CD is progressive and refractory, resulting in diverse complications. Recently developed biological agents are expected to provide better outcomes for patients with CD. Clinical decisions and the choice of biologics should be individualized based on inflammatory burden, convenience, cost, side effects, and compliance. The third Korean guidelines for the management of CD have been developed based on recently published highquality clinical data. They provide evidence-based recommendations for IBD physicians when using biological agents. We hope that these guidelines will help physicians accurately make decisions regarding the management of patients with CD, including those with perianal fistulas.
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
Koh SJ has received consulting fees from Daewoong Pharma. and Ferring Korea; speaking fees from AbbVie Korea, Ferring Korea, Daewoong Pharma., Janssen Korea, Pfizer Korea, Taejoon Pharma., Korea Pharma., Yuhan Pharma., and Takeda Korea. Hong SN has received a research grant from Celltrion; consulting fees from Celltrion, Takeda Korea, Janssen Korea, Eisai Korea, Ferring Korea; speaking fees and consulting fees from Celltrion, Takeda Korea, Janssen Korea, Eisai Korea, Daewoong Pharma., Ferring Korea, Pfizer Korea. Ye BD has served on advisory boards for AbbVie Korea, Celltrion, Daewoong Pharma, Ferring Korea, Janssen Korea, Pfizer Korea, Shire Korea, and Takeda Korea, has received research grants from Celltrion and Pfizer Korea, has received consulting fees from Chong Kun Dang Pharm., CJ Red BIO, Cornerstones Health, Daewoong Pharma, Kangstem Biotech, Korea United Pharm. Inc., Medtronic Korea, NanoEntek, and Takeda, and has received speaker fees from AbbVie Korea, Celltrion, Ferring Korea, IQVIA, Janssen Korea, Pfizer Korea, Shire Korea, Takeda, and Takeda Korea. Except for that, no potential conflict of interest relevant to this article was reported.
Data Availability Statement
Not applicable.
Author Contribution
Conceptualization: all authors. Data curation: Koh SN, Hong SN, Park SK, Ye BD, Kim KO, Shin JE, Yoon YS, Lee HS, Jung SH, Choi CH. Methodology: Choi M. Project administration: Kim JS. Resources: Hong SN. Supervision: Choi CH, Na SY, Kim JS. Writing - original draft: Koh SJ, Hong SN, Park SK, Ye BD, Kim KO, Shin JE, Yoon YS, Lee HS, Jung SH. Writing - review & editing: Koh SJ, Na SY. Approval of final manuscript: all authors.