Colonic oncostatin M expression evaluated by immunohistochemistry and infliximab therapy outcome in corticosteroid-refractory acute severe ulcerative colitis
Article information
Ulcerative colitis (UC) is a chronic relapsing remitting inflammatory disease of the colon. The lifetime risk of presentation with acute severe ulcerative colitis (ASUC) is 15% [1]. Patients with ASUC receive first line therapy with intravenous corticosteroids, however, approximately 30% have corticosteroid-refractory disease [2,3]. In this situation, rescue medical therapy options include the anti-tumor necrosis factor (TNF) monoclonal antibody infliximab (IFX) or the calcineurin inhibitor ciclosporin [2]. A significant proportion of patients fail to respond to IFX therapy with reported colectomy rates at 1 year of 35% [4]. Biomarkers which identify patients with corticosteroid-refractory ASUC, with a reduced likelihood of IFX response, would significantly advance clinical care for these patients.
Oncostatin M (OSM) is a member of interleukin-6 cytokine family [5]. OSM expression has been demonstrated to be increased in inflamed intestinal tissue from patients with moderately active UC compared with healthy controls [5,6]. In an analysis of 200 patients with inflammatory bowel disease (IBD), including 2 cohorts from phase 3 clinical trials of IFX and golimumab, high pretreatment tissue OSM expression was strongly associated with anti-TNF therapy failure [6]. In our study we sought to determine whether colonic tissue OSM immunostaining, on biopsies from pretreatment endoscopies, had utility as a biomarker of IFX treatment outcome in hospitalized patients with corticosteroid-refractory ASUC.
Patients admitted for management of ASUC to St James’s Hospital in Dublin, Ireland between 2011 and 2017 were identified retrospectively. The diagnosis of UC was made using established clinical, endoscopic and histological criteria. Patients were included where they had received at least one rescue IFX infusion in the context of intravenous corticosteroid-refractory ASUC, had undergone pretreatment endoscopic assessment and had documented follow-up. IFX was administered as per the standard protocol with induction therapy consisting of 5 mg/kg infusions at 0, 2, and 6 weeks. Adjustment of induction infusion intervals was undertaken at the discretion of the treating physician. Concomitant additional treatment with mesalamine and immunomodulators was administered as indicated. Accelerated IFX induction was defined as the administration of 3 induction infusions in less than 28 days. Baseline demographic and clinical data were collected for each subject. Sigmoidoscopies performed prior to IFX initiation were reviewed and an endoscopic Mayo subscore was documented. C-reactive protein (CRP)/albumin ratio was calculated by dividing CRP concentration by albumin concentration. The study was approved by the St James’s Hospital/Adelaide and Meath Hospital incorporating the National Children’s Hospital (approval No. SJH/AMNCH) Dublin, Research and Ethics Committee. This study is a retrospective study and so informed consent was waived.
Formalin fixed and paraffin embedded tissue specimens derived from biopsies collected at the time of pre-IFX sigmoidoscopy were identified. Four-micrometer sections were cut for immunohistochemistry studies and mounted onto slides. Sections were deparaffinized, rehydrated and unmasked using Trilogy pretreatment solution (Cell Marque, Rocklin, CA, USA). Sections were treated with a rabbit polyclonal antibody to OSM (Abcam, Cambridge, UK) at a 1:100 dilution. Samples were stained using the Vectastain Elite ABC HRP Detection Kit (Vector Laboratories, Burlingame, CA, USA). Colonic tissue without the addition of OSM antibody was considered negative control while sections from a colectomy specimen performed for severe colitis were used as positive controls. Staining was assessed at × 10 magnification. OSM immunoreactivity was assessed separately in the epithelial and stromal compartments using the following scoring variables: percentage staining (0%–100%); and staining intensity: 0 (absent), 1 (weak), 2 (moderate), and 3 (strong) (Supplementary Fig. 1). Two observers, blinded by to clinical outcome, performed immunohistochemical scoring for each case with scoring variable values considered to be the average score from both observers.
The study’s primary endpoint was the association between OSM immunostaining and requirement for colectomy and colectomy-free survival. Secondary endpoints included the association between OSM immunostaining and requirement for accelerated IFX induction, serum inflammatory markers levels and time to IFX discontinuation.
Continuous data are presented as medians and ranges, categorical data are presented as percentages. The distribution of epithelial and stromal OSM staining percentage positivity and intensity were dichotomized around median values to develop low and high OSM staining groups for each OSM staining score. The resulting groups contained different numbers of patients but were as close to equal as was possible to achieve. Differences between proportions were assessed using the chi-square test or Fischer exact test as appropriate. Mann-Whitney U test was used to compare continuous variables between groups. Related continuous variables were compared using Wilcoxon signed-ranks test. Follow-up was calculated as the duration of time to colectomy or to last known follow-up where the patient remained colectomy-free. Kaplan-Meier survival curves were constructed for time-based endpoints. Differences in survival between OSM staining groups were assessed using the log-rank test. P values are two-sided and P values of < 0.05 were considered statistically significant in all analyses.
Twenty-one hospitalized ASUC patients were included. Patient age at study inclusion was median 39.5 years (range, 21.2–81.8 years). Endoscopic Mayo score on study sigmoidoscopy was median 3 (range, 2–3). At the time of IFX initiation, 52% of patients (n = 11) were receiving concomitant 5-aminosalicylate therapy and 1 patient was receiving a concomitant immunomodulator (methotrexate). Follow-up duration was median 59.9 weeks (range, 0.6–327.3 weeks). Thirty-three percent of patients received an accelerated IFX induction regimen. Colectomy during study follow-up occurred in 7 patients (33%).
The distribution of OSM immunostaining in the study cohort was as follows (all values median [range]: epithelial staining percentage, 90% [62.5%–100%]; OSM epithelial staining intensity, 2 [1–3]; OSM stromal staining percentage, 62.5% [25%–82.5%]; and stromal staining intensity, 2 [1–3]). OSM epithelial staining percentage and intensity were significantly higher than OSM stromal staining percentage and intensity, P<0.001 and P=0.01 respectively.
Neither colectomy rates nor colectomy-free survival differed comparing epithelial or stromal high and low OSM immunostaining groups (Fig. 1, Supplementary Fig. 2). There was no significant difference in the requirement for accelerated IFX induction comparing high and low epithelial and stromal OSM immunostaining groups (data not shown). Survival-free of IFX discontinuation did not differ significantly between epithelial or stromal OSM immunostaining groups (data not shown). There was a trend toward an association between increased epithelial OSM staining intensity and higher CRP and CRP/albumin ratio. Comparing high and low OSM epithelial staining intensity groups, median CRP and CRP/albumin ratio were 55.5 mg/L (range, 1.8–258.0 mg/L) versus 15.7 mg/L (range, 1.1–70.0 mg/L) (P=0.056) and 2.1 (range, 0.1–11.7) versus 0.5 (range, 0.0–2.0) (P=0.056), respectively (Fig. 2).
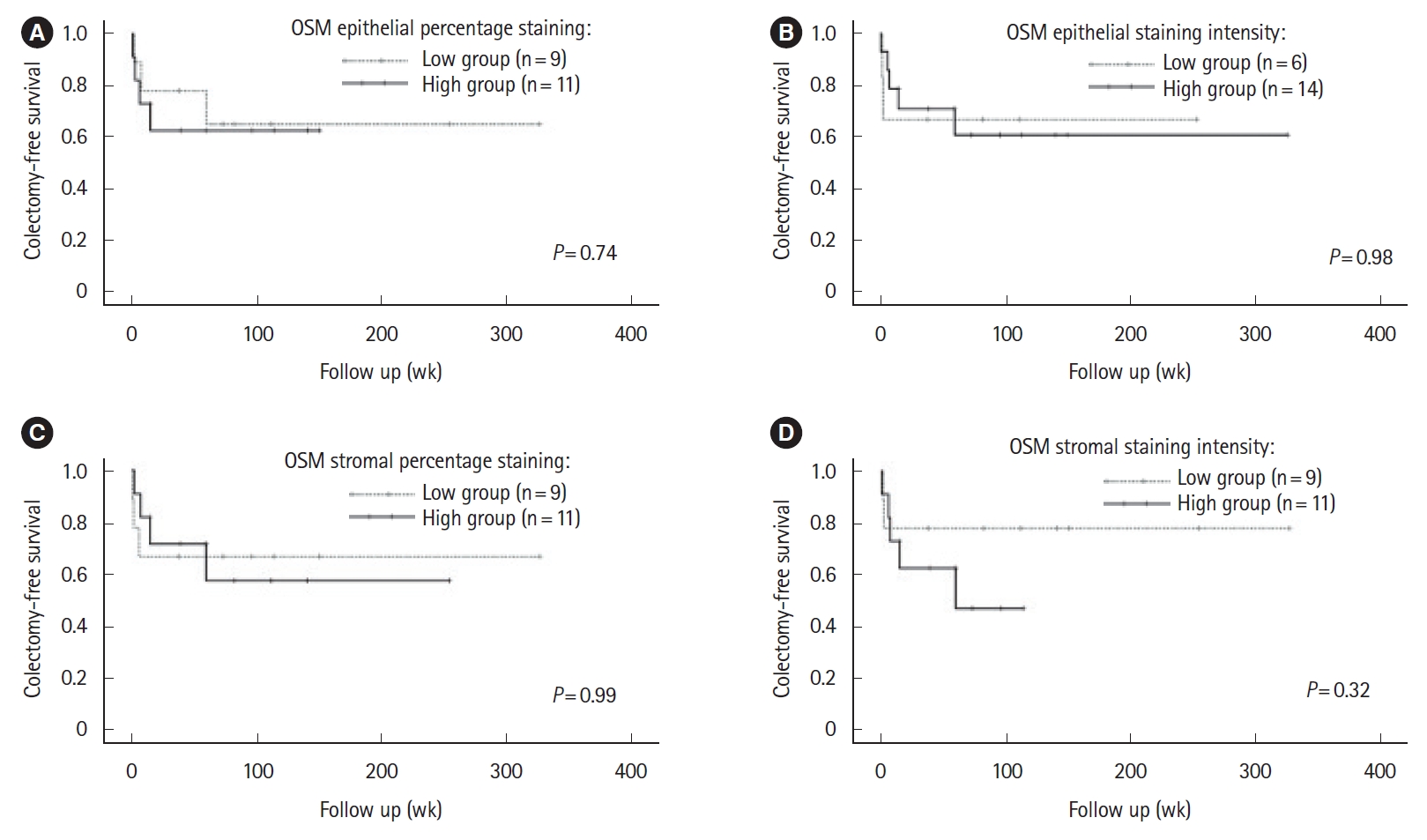
Association between oncostatin M (OSM) immunostaining colectomy-free survival. Kaplan-Meir survival curves describing colectomy-free survival in low and high OSM immunostaining groups. Panels show survival analysis for epithelial OSM percentage staining (A), epithelial OSM staining intensity (B), stromal OSM percentage staining (C), and stromal OSM staining intensity (D), respectively. One subject in the study cohort did not have data on time to colectomy available and was excluded from analyses.
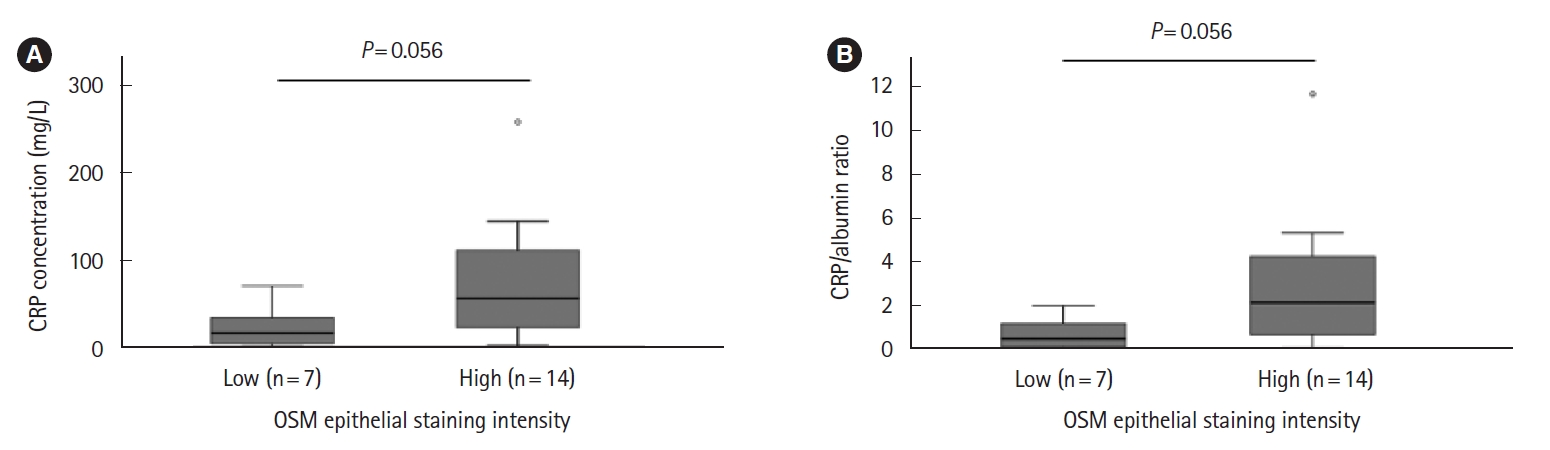
Association between epithelial oncostatin M (OSM) immunostaining intensity and C-reactive protein (CRP) (A) and CRP/albumin ratio (B).
In this report, we evaluate the association between colonic OSM expression, evaluated by immunohistochemistry, and the outcome of IFX rescue therapy in patients with ASUC. We did not demonstrate an association between colonic OSM expression and colectomy rates, requirement for accelerated IFX induction or time to discontinuation of IFX therapy.
Previous reports have demonstrated increased tissue OSM expression to be associated with intestinal inflammation in UC [5,6]. In concordance with these reports, our study demonstrated high OSM immunostaining in patients with ASUC, all of whom had moderate-to-severe mucosal inflammation on pretreatment endoscopic assessments. Higher OSM immunostaining was observed in the epithelial compared with stromal compartments which has not previously been observed to our knowledge. The differences between our findings regarding OSM localization and prior studies may relate to a number of factors. We studied patients with ASUC, a severe form of IBD, whose biology may differ significantly from milder forms of the disease which were studied in previous reports [5,7]. In addition, previous reports evaluated small number of patients. Further study is required to confirm the tissue localization of OSM in various IBD phenotypes. There was a relationship between increased epithelial OSM staining intensity and elevated serum CRP and CRP/albumin ratio, biochemical markers associated with adverse prognosis in ASUC [2,8]. This finding suggests the tissue OSM expressions levels may provide information on ASUC patients at risk of adverse outcomes.
The ACT1/2 (NCT00207688) and PURSUIT (NCT00487539) studies were phase 3 randomized controlled trials evaluating the efficacy and safety of IFX and golimumab as therapy for UC [9,10]. West et al. [6] previously demonstrated that OSM expression in pretreatment endoscopic biopsies from patients included in ACT1/2 and PURSUIT trials was strongly associated with non-response to anti-TNF therapy. Our study did not replicate the association between OSM immunostaining and outcome of rescue IFX therapy in ASUC patients. A small cohort of patients with ASUC was evaluated in our report and therefore the study may have been underpowered to detect an association between tissue OSM expression and outcome of IFX rescue therapy. The approach to the quantification of OSM levels in tissue differed between our study and that of West et al. [6] Our study utilized immunohistochemistry to characterize OSM expression in endoscopic biopsies, while the report by West et al. [6] evaluated tissue OSM mRNA expression. ACT1/2 and PUSRSUT studies included ambulatory patients with moderate-to-severe UC [9,10]. The population of patients evaluated in our study differed considerably, being hospitalized with severe disease, and it may be the case that OSM performs less well as a biomarker of anti-TNF response in this subpopulation of patients. The absence of an association between tissue OSM expression levels and anti-TNF therapy response in our study cohort should not be generalized to a broader outpatient UC population, given this association has been clearly demonstrated by West et al. [6] in large, well characterized cohorts with independent replication.
In summary, we did not demonstrate an association between pretreatment colonic OSM expression and the outcome of rescue IFX therapy in a small cohort of ASUC patients. Further studies, including larger patient populations, are required to definitively assess the utility of tissue OSM as biomarker of anti-TNF therapy response in patients with ASUC.
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability Statement
Not applicable.
Author Contribution
Conceptualization: Larkin J, MacCarthy F, Mehigan B, O’Sullivan J, Kevans D. Data curation: O’Connell J, Doherty J, Buckley A, Larkin J, Muldoon C, Ryan C, Kevans D, Cormican D. Formal analysis: Buckley A, Larkin J, MacCarthy F, Mehigan B, Kevans D, Cormican D. Investigation: Dunne C, Larkin J, Muldoon C, Ryan C, O’Sullivan J. Methodology: O’Connell J, Doherty J, Buckley A, O’Sullivan J. Project administration: O’Connell J. Resources: Mehigan B. Supervision: Hartery K, McKiernan S, O’Sullivan J. Validation: Buckley A, Hartery K, Ryan C. Visualization: Buckley A, McCormick P. Writing - original draft: O’Connell J. Writing - review & editing: O’Connell J, Buckley A, Hartery K, Larkin J, MacCarthy F, McKiernan S, Mehigan B, O’Sullivan J, Kevans D. Approval of final manuscript: all authors.
Others
The authors would like to acknowledge the support of Niamh Clarke in the conduct of this study.
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Fig. 1. Representative figure demonstrating oncostatin M (OSM) immunostaining in colonic biopsy specimens. High (A) and low (B) epithelial and stromal OSM immunostaining in a colonic biopsy specimen from a patient with acute severe ulcerative colitis (ASUC). (C) Absence of OSM immunostaining in the negative control.
ir-2021-00073-suppl1.pdfSupplementary Fig. 2. Oncostatin M (OSM) immunostaining in study cohort segregated by colectomy status. Histograms are displayed demonstrating OSM immunostaining score frequencies comparing acute severe ulcerative colitis (ASUC) patients who did not undergo colectomy (blue bars) and those who underwent colectomy (orange bars). (A) Epithelial percentage staining score distribution comparing ASUC patients who did not undergo colectomy and those who underwent colectomy. (B) Epithelial staining intensity score distribution comparing ASUC patients who did not undergo colectomy and those who underwent colectomy. (C) Stromal percentage staining score distribution comparing ASUC patients who did not undergo colectomy and those who underwent colectomy. (D) Stromal staining intensity score distribution comparing ASUC patients who did not undergo colectomy and those who underwent colectomy.
ir-2021-00073-suppl2.pdf