 |
 |
- Search
| Intest Res > Volume 22(1); 2024 > Article |
|
Abstract
Current evidence posits a central role for gut microbiota and the metabolome in the pathogenesis and progression of inflammatory bowel disease (IBD). Fecal microbiota transplantation (FMT) has been established as a means to manipulate this microbiome safely and sustainably. Several aspects of the technical improvement including pretreatment with antibiotics, use of frozen stool samples as well as short donor-to-recipient time are proposed to improve its response rates. Its efficacy in ulcerative colitis has been proven in clinical trials while data is emerging for CrohnŌĆÖs disease. This review describes briefly the biology behind FMT, the available evidence for its use in IBD, and the host, recipient and procedural factors which determine the clinical outcomes.
Inflammatory bowel disease (IBD) is a chronic inflammation of the gastrointestinal tract and broadly includes CrohnŌĆÖs disease (CD) and ulcerative colitis (UC). The pathogenesis of IBD is complex but is believed to involve an aberrant immune response to pathogenic gut microbiota in genetically predisposed individuals. The importance of gut microbiome, as reflected in the excreted fecal microbiome, is growing as we continue to decipher its language with novel molecular techniques [1]. The gut microbiome is huge, with 1013 to 1014 bacterial residents, and in the presence of disease is even more complex: microbes interact with each other and the host immune system, are affected by antibiotics or immunosuppressive drugs, and modulate the drugsŌĆÖ metabolism. Fecal microbiota transplantation (FMT) involves the transfer of fecal matter from a healthy donor into the gastrointestinal tract of a patient to restore a healthy microbial community. This review article aims to provide an overview of the dynamics of gut microbiome in IBD, the mechanism of action of FMT, technical and safety aspects of FMT and clinical outcomes following FMT in patients with IBD.
Awareness of changes in gut ecosystem in IBD is essential to understand the potential benefits of microbial manipulation therapies of which FMT is a prime example. These changes can be in the composition of gut bacteria, virome, mycobiome, and metabolome. The dynamics have a spatial distribution with changes in fecal microbiota being different from mucosal microbiome and crypt-specific microbiome.
Gut microbiome of patients with IBD, whether CD or UC, is characterized by reduced taxonomic diversity and phylumlevel decrease in Firmicutes and an increase in Proteobacteria abundance [2]. Even healthy individuals with IBD-susceptibility genes (high ŌĆ£IBD genetic risk scoreŌĆØ) have differences in microbiota from those without these susceptibility genes, such as a decrease in Roseburia genus responsible for acetate to butyrate conversion, that may precede the clinical onset of IBD [3]. It is unclear whether IBD produces dysbiosis or dysbiosis leads to IBD, however, several microbiota-altering exposures such as widespread antibiotic use, exposure to antibiotics in the first year of life, and consumption of a diet rich in fats, polyunsaturated fatty acids, proteins and low in dietary fiber, have been associated with incident IBD [4-8]. Initial studies found an association between IBD and infection with Mycobacterium avium paratuberculosis or adherent-invasive Escherichia coli, however, the data was inconsistent and therapeutic trials to eradicate these infections have not altered disease course [9-12]. Firmicutes phyla seem to play a central role in the anti-inflammatory microbial profile of a healthy population. Key members of this phyla include Faecalibacterium and Roseburia both of which have been found to produce anti-inflammatory metabolites, are depleted in patients with IBD, are repleted by FMT, and repletion correlates with response to therapy [13]. The evidence of this association between IBD and specific microbiota changes (e.g., abundance of Bacteroides and depletion of Firmicutes) has been strengthened by their association with several key aspects: aggressive disease [14], occurrence of complications, postsurgical recurrence, or pouchitis following colectomy for UC [15], and response to therapy with biological agents including antitumor necrosis factor agents [16,17] or anti-integrin agents [18]. However, the information obtained from microbiota analysis is limited in generalizability across regions due to the influence of genetics, diet and environment on the microbiome. The gut microbiome of Chinese and Western (from the Human Microbiome Project) IBD patients have marked differences with different predominant species even within specific IBD types [19].
Stark differences in the microbiome of IBD and non-IBD patients contribute to functional changes in the metabolic milieu, or metabolome. The metabolome is considered to be the route of interaction between the immune cells and microbiome through the production of pro-inflammatory or anti-inflammatory metabolites. Short-chain fatty acids (SCFAs), case in point, including butyrate, propionate, and acetate, are derived from the anaerobic bacteria-mediated breakdown of dietary fiber and modulate host mucosal cells by regulating cell proliferation and immune response [20]. Two G protein-coupled receptors (GPR), GPR41 and GPR43, and regulatory T cells (Treg) cells are activated by SCFAs [21]. SCFAs promote the accumulation of Treg CD4 cells in the colonic mucosa and protect against colitis [22]. Similarly, CARD9 deficient mice are at an increased risk of developing CD due to reduced microbial production of tryptophan, and the risk is attenuated by supplementing with tryptophan metabolizing Lactobacillus species [23]. Metabolomic and metagenomic profiling of the IBD fecal samples indicate enrichment of certain metabolites (bile acids, sphingolipids) and depletion of others (tetrapyrroles, triacylglycerols), indicating increased activity of functions such as those allowing adaptation to oxidative stress in IBD [1]. The microbiome and metabolome profiles could differentiate IBD from non-IBD stool samples with an area under the curve of 0.92. Vich Vila et al. [24] analyzed fecal metabolome and metagenomics in 424 patients with IBD. They found that the fecal metabolites in IBD were characterized by lower levels of saccharolytic fermentation derivatives and increased metabolites from proteolytic fermentation. Higher sphingolipids, ethanolamine, and primary bile acids characterized the gut signature of patients with IBD versus controls and the ratio of sphingolipid-Lurobilin carried high accuracy (area under the receiver operating characteristic curve = 0.85) to differentiate the two. Importantly, their findings supported that gut microbiota composition is the main determinant of fecal metabolite content, compared with patient lifestyle, genetics, or clinical phenotypes, highlighting the centrality and distinct role of gut microbiome. Machine learning algorithms incorporating clinical, microbiome, and metabolome data, have been designed to predict response to anti-integrin therapy in IBD with high accuracy (area under the curve = 0.872) [18].
The fungal and viral components of the microbiota are important but have been neglected due to difficulty in sequencing techniques. Fungal mycobiome is usually sequenced using the ŌĆ£universal fungal barcode sequenceŌĆØ of the ribosomal RNA called internal transcribed spacer [25]. Ascomycota and Basidiomycota are the most prevalent taxa in the mucosa of healthy subjects. The abundance of the fungus Candida tropicalis is higher in patients with familial CD compared to non-affected relatives, and it positively correlated with levels of anti-Saccharomyces cerevisiae antibodies that are purported to be against Candida antigens [26]. Further, the abundance of C. tropicalis correlated with that of Serratia marcescens and E. coli, suggesting that these organisms interact in the gut and a biofilm formed from these 3 organisms was thicker than those of any single- and double-species biofilms along with morphological changes in hyphae formation when the 3 organisms coexisted. A probiotic combination of Saccharomyces, 3 bacterial species, and amylase was effective in preventing and treating this biofilm formation [27]. Another report from Japan found similar alpha diversity in patients with IBD and controls, but increased abundance of Candida in CD compared to healthy controls and UC [28]. Two studies linked different fungal genera (Debaryomyces and Malassezia) with mucosal inflammation in CD and demonstrated their pro-inflammatory potential in animal models [29,30]. Evidently mycobiome holds more relevance in CD than UC.
Virome signature interpretation is even more difficult-longitudinal analysis has shown that the virome is less stable and shows interpersonal variability in viral taxa, frequent fluctuation of taxa even within an individual, and vast fluctuations in the abundance of temporal viruses [31]. An in-depth analysis of free virions enriched intestinal preparations showed significant enhancement of Caudovirales bacteriophages in both CD and UC [32]. The mucosal virome is distinct from the fecal virome and houses certain phages that escape detection in stool samples [33]. The alpha and beta diversity of viromes is unable to differentiate IBD from controls, unlike the bacteriome.
The fecal microbiome is the usual source of microbiome information, being easily accessible and noninvasive, and allowing longitudinal sampling. However, there appear to be additional microbiome strata that line the mucosal lining, as well as the crypts. Recent advancements in single-cell and spatial transcriptomics have enabled us to characterize these niches [34]. The mucosa-associated microbiome and crypt-associated microbiomes are ŌĆ£deeperŌĆØ and thus closer to the host epithelial lining and immune cells. They have been found to have distinct microbial profiles from the fecal microbiomes. The mucosa-associated microbiome is more ŌĆ£abnormalŌĆØ and differentiates better from controls than the fecal microbiome, and has a uniform increase in Proteobacteria and reduction in Firmicutes regardless of IBD type and severity [35,36]. The mucin of the epithelial lining in UC has a lower degree of sulphation and correspondingly lower abundance of sulfated-mucin metabolizing species Desulfovibrio [37]. Early studies found that only a few crypts in IBD patients are colonized by bacteria unlike acute self-limiting colitis, however, these used fluorescent in situ hybridization techniques that have been since surpassed by laser-microdissection of tissues and 16S rRNA gene sequencing [38]. The composition of crypt-associated microbiota (CAM) in health, as well as disease, is dominated by aerobic members, which can be attributed to higher oxygen tension in the crypts [39]. Microbiome composition analysis showed mucosa-associated microbiome being dominated by members of phyla Firmicutes (45%), followed by Bacteroidetes (26%), Proteobacteria (16%), and Actinobacteria (6%), while CAM comprised predominantly of aerobic members of Actinobacteria (54%) and Proteobacteria (38%). Dysbiosis has been reported in salivary samples in IBD, and some strains of Streptococcus are shared in saliva and fecal samples from patients with IBD [40]. Atarashi et al. [41] elegantly demonstrated the potential pathological role of ectopic oral microbial species (Klebsiella) when they colonized the gut in gnotobiotic mice, and drive intestinal inflammation via the TH1 cell pathway. They hypothesized that the oral cavity may serve as a reservoir for potential intestinal pathobionts.
FMT involves transferring colonic contents rich in bacteria from a ŌĆ£donorŌĆØ without major disease and an assumed ŌĆ£healthyŌĆØ microbiota, to a patient with IBD whose microbiota is ŌĆ£unhealthyŌĆØ and possibly contributes towards ongoing inflammation. FMT aims to restore a healthy microbial community in the gut which in turn may reduce inflammation and restore gut homeostasis. The exact mechanism of action of FMT in IBD is not yet fully understood, but it is believed to involve a combination of direct and indirect effects on the host immune system and gut microbiota (Fig. 1).
It serves to maintain epithelial integrity and reduce bowel permeability by increasing the production of SCFAs, especially butyrate and also restores immune dysfunction by inhibiting Th1 differentiation, activity of T cells, leukocyte adhesion, and production of inflammatory cytokines [42]. The main advantage of FMT over probiotics is its speed-it rapidly provides healthy saprophytic bacteria to the site of inflammation. The response to FMT is contingent, at least partly, on its ability to ŌĆ£correct dysbiosis.ŌĆØ Most patients who respond clinically to FMT in trials have been shown to have increased alpha diversity, reduced pretreatment Fusobacterium and Escherichia species, consistent enrichment posttreatment with Firmicutes bacteria (Roseburia and Eubacterium species), and an increase in biosynthetic pathways for SCFAs and secondary bile acids production in the recipient microbiome [43,44]. An interesting study correlated FMT success with the transfer of IgA-coated Odoribacter splanchnicus, which mediated immune cell protection from colitis [45]. At least half of FMT studies show an increase in alpha diversity of the host, and at least a third show an increase in beta diversity with a trend towards respective donors [44]. A clinical trial administering FMT in active UC has shown enhancement and reduction in specific CAM genera as well as their respective negative and positive correlations with fecal calprotectin levels and clinical/endoscopic disease activity scores, thus highlighting the potential link of CAM with the course of UC and its FMT-mediated restitution [39]. Perhaps unsurprisingly, autologous FMT has not been found useful in IBD due to the dysbiotic nature of the patientŌĆÖs microbiome [46]. The contribution of modulation of the immune system by FMT remains to be explored. As a proof of concept, a patient with treatmentrefractory aggressive UC received FMT based on the donor feces that generated the least cytokine response from rectal lymphocytes obtained from tissue biopsy [47]. There was excellent engraftment and clinical response, indicating that minimal immune response to donor microbes allows engraftment.
There are several host, recipient, and procedure-related factors which determine the outcome of FMT therapy in IBD. There is great heterogeneity in techniques of FMT across centers worldwide, over time, and between different diseases. However, many of these issues are being addressed gradually through consensus and a uniform protocol is slowly evolving. All these issues have been explored here (Fig. 2).
There is a stringent screening of the donors for viral pathogens such as hepatitis A, B, and C viruses, human immunodeficiency virus, Ebstein-Barr virus, and cytomegalovirus, severe acute respiratory syndrome coronavirus 2, bacterial pathogens such as Clostridioides difficile, drug-resistant bacteria particularly Shigatoxin-producing E. coli and enteropathogenic E. coli using antimicrobial resistance genes, and parasitic infestations [48,49]. Appropriate screening is often time and resource intensive with around 90% of prospective donors failing screening, half of them due to lack of follow-up [50]. A complete list may be accessed through several consensus guidelines [51,52].
The choice between frozen versus fresh feces being used for FMT is an area of ongoing debate. Frozen feces can be stored at -80┬░C for up to 2 years, allowing the setup of ŌĆ£stool banksŌĆØ at several FMT centers and ready access to clinicians for use. This allows testing of each sample prior to administration and it has been recommended for this safety factor [52]. On the other hand, freezing of feces is known to impact microbiome composition. It leads to a 4-fold reduction in living bacterial cells as detected by flow cytometry (70% to 15%), similar alpha diversity, and definite differences in beta diversity on principal coordinates analysis from fresh fecal samples [53]. Prolonged frozen storage leads to an increase in Firmicutes to Bacteroidetes ratio, as some Gram-negative species are depleted during storage and some hypothesize that trials in which frozen FMT was found to be effective also depend on this phenomena of selective depletion of administered Bacteroidetes. A network metaanalysis of 8 studies in patients with recurrent C. difficile infection (CDI) found similar efficacy in fresh versus frozen FMT (93% vs. 88%, P= 0.18) and concluded that the trend towards improved efficacy with fresh FMT was offset by safety, accessibility, and practicality of frozen FMT [54]. Similar findings have been observed in UC from meta-analyses of 22 studies where the efficacy of fresh (34.4%) versus frozen (46.8%) was similar [55]. However, an understanding of the biological effect of freezing on microbiome and its effect on the sustenance of response remains to be seen.
Washed FMT is a technique developed in Chinese FMT centers using an automated washing machine GenFMTer (FMT Medical, Nanjing, China). It includes additional steps of filtration, centrifugation and resuspension three times in an attempt to retain intact bacteria and remove fecal particles, parasite eggs and fungi. Chinese databases found a reduction in adverse events due to FMT from 38% to 12% upon switching from conventional FMT preparation practices [56]. Intraperitoneal injection of washed FMT into mice after increasing number of centrifugation cycles showed a reduction in viruses, and pro-inflammatory metabolites (leukotrienes, prostaglandin G2, corticosterone), and prevented death of these experimental mice [56]. It has been shown to not impair efficacy in either CD or UC, however, has not been validated in other countries [57]. Another technique fecal filtrate transplantation instead removes intact bacteria and only transfers bacterial debris, metabolites, and oligonucleotides [58]. It has been utilized successfully in CDI and is also being evaluated in UC in a clinical trial [59].
Anaerobic preparation of FMT involves processing frozen FMT under anaerobic conditions, at the steps of homogenization, freeze-thaw cycles, and the time between defecation till transplant preparation, with the benefit of preservation of obligate anaerobes from the donor. Costello et al. [60] found that all microbes associated with treatment response of FMT in UC were anaerobes (mostly obligate anaerobes). Obligate aerobes (such as Faecalibacterium prausnitzii and Bacteroides fragilis) tend to decrease with mixing during preparation but anaerobic preparation mitigates this decrease, while facultative anaerobes (such as Enterobacteriaceae, Streptococcus) proliferate during mixing [61]. Commercially available systems (such as anaerobic chamber Bactron IV Work Station, USA) use a nitrogen (85%) + hydrogen (10%) + carbon dioxide (5%) atmosphere to maintain anaerobic conditions. Chu et al. [62] used propidium monoazide-based sequencing to differentiate viable and dead bacterial cells and assess the effect of various preparation methods. They found that oxygen exposure degraded fecal microbial composition (particularly abundance of F. prausnitzii), while freeze-thaw cycles and lag time between donor defecation and transplant preparation had smaller effects. Another group found drastically lower viability (19%) in aerobic preparation under ambient air, compared to anaerobic preparation (50%) [63]. A shorter duration between defecation by donor and administration of FMT has been found in those who respond to FMT versus nonresponders (median 2.1 hours vs. 2.6 hours, P= 0.002) with a drastic reduction in response rate at time gap > 3 hours [64]. Consensus guidelines suggest the use of fresh stools within 6 hours, and frozen processed stools used within 6 hours of thawing, in an effort to preserve anaerobic species [51]. Thus, anaerobic preparation is superior in composition of beneficial bacteria and should be practiced consistently, but its direct impact on clinical outcomes remains to be established.
Pretreatment with antibiotics improves engraftment of donor FMT. Weekly sequencing of fecal microbiota during an 8-week course of FMT demonstrated that higher alpha diversity and donor engraftment strongly correlate with clinical response [65]. Additionally, ŌĆ£post-antibiotic pre-FMTŌĆØ time point alpha diversity, i.e., residual diversity after antibiotic therapy, correlates well with both engraftment and subsequent clinical response. This is in contrast to the lack of independent efficacy of antibiotics or probiotics in typical non-fistulizing CD and UC [66]. Initial studies in mice compared pretreatment with antibiotics, colonic lavage or no pretreatment prior to FMT and found superiority of the antibiotic regimen. They demonstrated that this treatment allows colonization by the xenomicrobiota (from the donor human feces), by eliminating antibiotic-sensitive bacteria and generating niches for colonization [67]. Ishikawa et al. [68] reported using triple antibiotics (amoxicillin, metronidazole, and fosfomycin) prior to FMT, with the intent to eradicate harmful Bacteroidetes species. A systematic review of 28 articles (including 6 randomized trials), in which 11% of the patients were pre-administered antibiotics, found an improvement in response rate (82% vs. 58%) and remission rates (66% vs. 31%) with antibiotic pretreatment that was consistent with an increase in alpha-diversity and enrichment with SCFA producing anaerobes [44]. Higher baseline microbiota diversity is also linked with greater response rates, suggesting that the dysbiosis can potentially exceed the capability of modulation with FMT [69].
Bowel preparation (with agents like polyethylene glycol) is commonly used prior to FMT, regardless of whether FMT is administered via colonoscopy, nasoduodenal tube or gastroscope [51,70]. It aids cecal intubation, removal of vegetative cells, and allows engraftment of transplanted microbes [71]. The lavage impact does not alter the gut microbiota independently over long periods but may have short-term reduction in the total bacterial abundance of fecal microbiota as well as mucosa-associated microbiota [72-74]. No controlled studies have assessed its clinical impact on FMT in IBD but data from recurrent CDI suggest that poor bowel preparation is associated with failure of FMT [75].
Typically fecal matter is converted to a slurry and administered via either one of several routes: during colonoscopy, during gastroscopy, via nasogastric or nasojejunal tube, as enemas, or as oral ingestion of capsules. FMT administered via nasogastric tube was as effective as colonoscopy in treating recurrent CDI in a pilot randomized controlled trial (RCT) [76]. Similarly, administration through a gastroscope or colonoscope did not affect efficacy or adverse events in a trial of CD [77]. Meta-analyses have suggested higher response rates with colonoscopic instillation instead of upper gastrointestinal administration (31% in 137 patients vs. 8% in 42 patients) [78]. The only negative RCT on FMT in UC utilized the nasoduodenal route, suggesting that the lower gastrointestinal route (colonoscopic or rectal enema) would be preferred in UC [79]. The efficacy of oral capsules in both induction and maintenance of remission in mild to moderate UC was demonstrated in the LOTUS trial [80]. Oral capsules as FMT have thus far been limited to recurrent CDI but not IBD [81]. A large trial and subsequently non-randomized study proved non-inferiority of capsulized frozen FMT and colonoscopic administration in preventing recurrent CDI with roughly half the adverse event rate [82].
The dose of donor feces administered per session and cumulatively is probably one of the most heterogeneous aspects of the practice of FMT. As little as 30 g, and as much as 600 g, have been utilized in clinical trials of IBD. Zhao et al. [55] identified 275 g as the median dose used in the 29 studies they included in their meta-analysis. Studies using higher doses of donor feces ( > 275 g) were associated with higher response rates (52% vs. 30%) than those using lower doses. Similarly, subgroup analysis by Wei et al. [83] found benefit of FMT in induction of remission of UC only when the cumulative dose was higher than 300 g (risk ratio [RR], 1.85; 95% confidence interval [CI], 1.22-2.83) but not in lower doses. To achieve these doses, several sessions may be required, and there may be differences in optimum doses for induction versus maintenance of remission in IBD. A higher load (60 g vs. 30 g) of infused donor feces led to a greater reduction of irritable bowel syndrome (IBS) symptoms (89% vs. 76%) following FMT [84]. There are fallacies with using fecal weight as a ŌĆ£currencyŌĆØ and there is a need for developing ubiquitous and validated measures to assess the dose of active bacteria being administered.
Repeated sessions allow repeated exposures to the donor microbiome and allow an opportunity for donor microbial engraftment, which is correlated closely to clinical respons [44,85]. This effect is seen with repeat sessions independent of the disease and mimics dose escalation of conventional therapy. Response rates for repeat FMT in recurrent CDI were superior (91% vs. 84%) with a consequently lower number needed to treat (1.5 vs. 2.9) in a large meta-analysis [86]. In a small study of patients with IBS, those who did not improve with FMT initially had an excellent response following a repeat session with double dose of transplant feces [87].
A meta-analysis by Mocanu et al. [44] found higher pooled response rates (70% vs. 53%) and remission rates (43% vs. 30%) to FMT in those given repeated doses, as compared to a single dose. Regimens applied to patients with IBD have wide variability, and the following have been used in various clinical studies: daily enemas [88], alternate day enemas or nasojejunal or colonoscopy [89], initial colonoscopy followed by twice weekly enemas [60], weekly colonoscopies [90,91], 2 weekly colonoscopies [69], and some have utilized regimens similar to those for biological agents with an induction and maintenance phase [92]. Ren et al. [85] evaluated ŌĆ£low intensityŌĆØ FMT sessions for two sessions (0 and 2 months) with a single super donor and found remission rates of 21% and 55% after the first and second FMT sessions. No comparison is available between the different regimens due to small number of available studies, and differences in FMT composition and patient populations receiving the therapy.
Importantly, there is no single optimum way to perform FMT and each institute should establish standard operating procedures to minimize heterogeneity in technique. Centers catering to patients with IBD and establishing FMT in their practice should set up stool banks to provide a steady supply of frozen stool for FMT.
An easy workaround for enriching the microbial diversity of donor fecal samples is to pool multiple donors. Pooling of fecal samples proposes to provide a wider spectrum of microbes such that ŌĆ£deficienciesŌĆØ of the dysbiotic recipient microbiome can be resolved. Mathematical modeling based on the RCT by Moayyedi et al. [90] suggested that pooling of 2 or 3 donors can increase remission rates in UC [93]. Multi-donor or pooled FMT have been compared with single donor FMT in a meta-analysis of 14 studies that indicate the superiority of the former (RR, 2.31; P= 0.04) in terms of treatment response in IBD [94]. Another meta-analysis that did not detect any effect of single or pooled donors attributed this to the possibility of ŌĆ£super donorsŌĆØ in those receiving from a single donor. Importantly, head-to-head comparisons are lacking since institutes typically use a uniform policy for sourcing donor samples, and such trials are expected in the future.
The donor fecal microbiome is important in determining response to therapy. Focus, thus far, during the selection of donors has been on safety-particularly preventing transmission of pathogenic or virulent organisms or genes. Recurrent CDI required the replacement of healthy gut microbiome to replace niches for C. difficile and thus trials were not focused on selecting donors for their effect on efficacy. However, in contrast to CDI, there is emerging evidence linking properties of donor microbiome with clinical outcomes in IBD. In an early trial reporting the efficacy of FMT in inducing remission in UC, remission was attained in 9 patients (24%) in the FMT arm, 7 of whom received FMT from a single donor [90]. This donor, characterized by enrichment for the family Lachnospiraceae and the genera Ruminococcus, showed much higher remission rates (39% vs. 10%). Further, there was a clear association between achieving a microbiome similar to the donor at week 6 and achieving clinical remission after FMT. This suggested the phenomena of ŌĆ£super donorsŌĆØ: donors whose fecal microbiota characteristics greatly improve clinical response to FMT [95,96]. A smaller donor-recipient age difference (< 10 years) was associated with improved maintenance rates in a trial of FMT following antibiotics in UC [97]. A prime parameter of the donor fecal microbiota responsible for this appears to be high alpha diversity and absolute richness. A single-arm study utilized a single donor with high microbial diversity and fecal butyrate concentration, considered to represent healthy gut microbiome, for FMT to 10 pediatric patients with recurrent CDI and found complete response in all patients at 10 weeks, as well as a sustained increase in phylogenic diversity of recipient microbiome over this period [98]. Similarly, in UC patients undergoing FMT, higher alpha diversity was associated with better recipient outcomes in 2 trials [69,43]. Longitudinal analysis of 2 donors from the LOTUS trial identified long-term microbiome stability and donor species evenness as novel factors associated with FMT success [99]. There have been several individual microorganisms that have been proposed to influence response, but these findings have been limited to individual trials and few could be replicated in other studies, suggesting a need for broader assessment than individual organisms [95]. A metagenomic analysis of 14 public databases of FMT trials in CDI and IBD identified that donor fecal microbiome with donor/p phenotype, indicating Prevotella as the dominant taxa, were associated with a greater response to FMT versus donor/b phenotype (65.1% vs. 40.1%), rich in Bacteriodetes [100]. Clinical response to these donor phenotypes correlated well with an increase in recipient alpha diversity. However, Olesen and Gerardin [101] reevaluated the statistical evidence related to super donor phenomenon in studies on FMT in IBD and suggested uncertainty on the magnitude of donor effect and the need for larger studies specifically designed to assess donor phenomena. When possible, donors should be unrelated to the recipient.
As important as selecting appropriate donors is identifying those who will benefit from FMT, vis-├Ā-vis several options for pharmacotherapy. A prospective cohort treating patients with moderate to severe UC analyzed clinical predictors of response to FMT: younger patients, left-sided colitis disease extent (E2 disease, vs. either proctitis or pancolitis), and endoscopic Mayo score of 2 were associated with remission, while severe disease (Mayo Clinic score Ōēź 2), Mayo endoscopic score 3, and pancolitis (E3 extent) were associated with failure of therapy [92]. A systematic review of 25 studies analyzed response predictors to FMT in IBD [102]. Donor fecal microbiome SCFA production, higher alpha diversity, and greater abundance of Bacteroidetes and Clostridia clusters were associated with clinical response. Recipient clinical parameters of young age, less severe disease, and shorter duration of disease, and microbiome characterized by higher fecal species richness, greater abundance of Candida, and similarity to donor profile were also significant predictors.
Current technology and computational setups allow a detailed analysis of the gut microbiome. However, it is not ready for clinical application because of the involved cost and turn-around time. Retrospective analysis of donor-recipient matching provides insights into the deficiencies of the diseased microbiome that are being filled by the donor microbiome. He et al. [100] developed a machine learning model for selecting donors based on recipients (enterotype-donors selection, EDS) and validated this model on a population of 42 patients with IBD. They found 93% clinical response in the patient-donor pairs who could be matched with the model and 37% in those who were not selected by the model. An analytical-hierarchy based donor-recipient matching model was proposed by Zhang et al. [103] based on sequencing data from 350 patients with UC receiving FMT. Important indicators for this model included alphadiversity (28% of weight) and presence of beneficial taxa and metabolic pathways (24% and 22% of weight), and this model showed favorable classification ( > 70%) for FMT effectiveness in 2 prior trials. A matching strategy based on ex-vivo response assessment of patientŌĆÖs rectal immune cells to candidate donor specimens has been explored in a case report [47].
Engraftment of donor fecal species into the gut of the recipient, and correction of dysbiosis, is the basis of FMT therapy. However, the degree to which new taxa are added and existing taxa are displaced from the microbiome pool is variable. Patients who responded well to FMT and achieved remission had greater diversity both at baseline as well as post-FMT, compared to those who did not respond [43]. Patients in remission after FMT had enrichment of Eubacterium and Roseburia post-FMT and increased levels of SCFA biosynthesis and secondary bile acids, versus enrichment of Fusobacterium, Sutterella, and Escherichia species and increased levels of heme and lipopolysaccharide biosynthesis in those who did not respond. A recent study conducted by our group explored the effect of FMT on the composition of fecal and mucosal microbiota and metabolome in patients with UC [104]. An 8-week pooled multi-donor FMT in our patient cohort mediated enhancement of alpha diversity, with increased abundances of beneficial gut microbial taxa such as Eubacterium, Alloprevotella, Blautia in the fecal microbiota and Alistipes, Eubacterium in the mucosal microbiota. These beneficial taxa have previously been reported to be active SCFAs and indole-based AhR ligand producers and bile acid transformers. These shifts in abundances were coupled with a reduction in abundances of pathobionts such as Pseudomonas, Streptococcus, Enterococcus, Klebsiella, and Staphylococcus. As seen here, there is semblance in the bacterial species and metabolite changes post-FMT across 2 different cohorts.
Several thousands of patients with UC have undergone FMT as part of clinical trials as well as patient care. Large meta-analyses have shown the efficacy of FMT in UC, and clinical trials for this indication are listed in Table 1 [60,79,80,90,91,105-113]. El Hage Chehade et al. [114] published their meta-analyses limited to double-blind placebo-controlled trials for induction of remission in UC and found a good composite clinical and endoscopic response with odds ratio (OR) of 4.1 (95% CI, 2.2-7.7). All included trials included patients with mild-moderate UC based on Mayo score between 3 to 10, and the longest duration of follow-up was 3 months. Subgroup analyses showed no significant influence on the efficacy of several parameters that are discussed above: fresh versus frozen FMT, single versus pooled donor, pretreatment with antibiotics, bowel lavage, concomitant antibiotic therapy, and route of FMT delivery. Zhao et al. [55] included both cohort studies (n = 30) and randomized clinical trials (n = 7) in their meta-analyses and found complete remission response rates of 35.9% in uncontrolled studies and OR of 3.39 (95% CI, 2.2-5.2) in controlled cohort studies and RCTs. Serious adverse event rates were low but significant (5.9%). Cochrane Society analyzed 12 RCTs published till 2022 and found a RR of induction of remission in UC to be 1.79 (95% CI, 1.13-2.84) but was not clear on its role in the maintenance of remission of active UC after induction (2 trials: RR, 2.97; 95% CI, 0.26-34.42) [115]. A recent network meta-analysis compared available pharmacotherapy and FMT for induction of remission in patients with UC [116]. Four of 19 included articles were trials for FMT, and they found a pooled OR of 2.8 (95% CI, 1.4-5.8) for remission compared to placebo-statistically similar efficacy as other biological agents. Overall efficacy ranking from highest to lowest was infliximab, tofacitinib, ustekinumab, FMT, and least vedolizumab and adalimumab. As of this review, consensus statements recommend providing FMT as part of clinical trials as there is insufficient evidence for routine clinical use [52].
Long-term follow-up of trials in FMT show that clinical response is not sustained after induction, indicating that additional microbiome manipulation is required to maintain the response [111,117]. Role of FMT in the maintenance of remission in UC has been explored in a randomized sham-controlled trial by Sood et al. that included patients with UC in remission and found higher rates of 48-week endoscopic (58% vs. 27%, P= 0.03) and histological (45% vs. 16%, P= 0.03) remission in those treated with 8 weekly FMT compared to placebo, although it did not meet primary outcome of clinical remission (87% vs. 66%, P= 0.11) [112]. Lahtinen et al. [113] randomized 48 patients with UC to a single session of FMT versus autologous transplant via colonoscopy (control) and found no difference in remission rates at 12 months (54% vs. 41%, P= 0.66), although quality of life scores were worse in FMT arm. This lack of efficacy is likely due to providing a single session of FMT versus multiple sessions.
Twenty percent of patients with moderate to severe UC remain steroid-dependent on follow-up, and FMT has been evaluated for the treatment of this subgroup in an attempt to avoid steroids and related adverse effects. Cui et al. [118] treated 15 patients of steroid-dependent UC with step-up FMT and 57% (n = 8) achieved clinical response and were able to discontinue steroids, and these patients were likely to have a change in their microbiota composition towards the donor. A real-world prospective cohort analysis of 49 patients with steroid-dependent UC were treated with 4-weekly multisession FMT through colonoscopy and 75% patients achieved clinical response at 24 weeks and could withdraw steroids [119]. A small clinical trial utilized FMT for induction, maintenance and rescue therapy in 27 patients with steroid-dependent UC, 13 of whom remained in remission at 24 weeks after induction with 3 fortnightly sessions of FMT [120]. Ten of 13 patients eventually relapsed at a median duration of 15 months, and 40% of these relapses were managed with rescue FMT alone. While these results are promising, no patient who was on steroids at the time of recruitment (surrogate for steroid-dependent disease) to the RCT by Paramsothy et al. [111]. achieved clinical remission with FMT. Thus, this population of steroid-dependent UC patients is positioned to be important stakeholders for future trials of FMT in UC.
Diet has been an important modulator of gut microbiome and hence it would be an attractive strategy to modify diet along with FMT [121]. We performed an open-label RCT in which we randomized 66 patients with mild-moderate UC to a combination of FMT and anti-inflammatory diet (AID), compared to optimized standard medical therapy [91]. There were high response rates in the FMT-AID arm with over two-thirds of patients having a clinical response at 8 weeks (OR, 3.5; 95% CI, 1.3-9.6). Further, the response to induction in the FMT-AID arm was maintained with AID alone in 25% of patients (vs. 0% in SMT, P= 0.007). The AID consisted of a combination of dietary constituents that have been found to ameliorate gut inflammation in pre-clinical models and epidemiological studies-mainly, avoidance of gluten-based grains, dairy products, processed meats, refined sugars, and increasing amount of fruits and vegetables in diet [122]. Another small trial was stopped due to futility after the recruitment of 62 patients when UC exclusion diet (UCED) was found to be superior to FMT with or without UCED based on higher clinical remission and mucosal healing [106]. This suggests the need for further head-to-head comparison of UCED versus FMT and as adjuncts.
There have been emerging reports on the use of FMT in patients with acute severe UC (ASUC). A patient who developed ASUC on a background medical therapy comprising of infliximab, azathioprine and mesalamine, and did not respond to steroids by day 3 was provided FMT and had clinical as well as endoscopic remission on follow-up [123]. Another case report depicted failure of FMT as a salvage therapy in a 3-year-old patient with ASUC who eventually required colectomy [124]. Microbiome analysis confirmed the lack of alteration in gut microbiome in this patient despite FMT, possibly explaining the lack of response.
A systematic review analyzed 228 patients from 11 cohort studies and 1 randomized trial that evaluated the efficacy of FMT in the induction of remission in CD [125]. They found 57% of patients achieved clinical remission within 2 to 4 weeks after FMT and significantly reduced CrohnŌĆÖs Disease Activity Index (CDAI) scores within 4 to 8 weeks after FMT. Three of the 12 included studies restricted recruitment to patients with L2 (colonic) disease, possibly due to positive experience in UC. Subgroup analyses found that pre-FMT treatment with antibiotics led to improved remission rates. Further, after FMT there was an increase in microbiome alpha diversity (Shannon index) and a shift towards donor microbiome profile. Yang et al. [77] only included patients with active (clinical disease activity, CDAI scores > 150) colonic disease in their clinical trial. They administered FMT either via gastroscopy or colonoscopy in half patients each. Baseline CDAI scores (mean) were 275 and 290 in the gastroscopy and colonoscopy groups, respectively, with an overall clinical remission rate (CDAI < 150) in 66% of patients which was similar in both groups. Importantly, no clinical trial in CD till date, nor any cohort, has compared FMT in CD with placebo [115]. Thus there is an urgent need for placebo-controlled clinical trials of FMT in CD, and results of recently completed trials are awaited (Clinicaltrials.gov NCT 03078803) [126].
A pilot RCT included 17 patients with colonic or ileocolonic CD and after attaining remission of flare with steroids, were randomized to either FMT or placebo for maintenance of remission [127]. Remission rates at 10 weeks were 87% and 44%, and at 24 weeks were 50% and 33% in the FMT and placebo arms respectively, but the study was underpowered for this comparison (RR for remission at 24 weeks 1.21; 95% CI, 0.36-4.14). Larger controlled studies are needed to ascertain the potential of FMT for CD, including both colonic and ileal disease.
There are several reasons why FMT has more consistent benefits in patients with UC while studies in CD have shown mixed results. Firstly, colonoscopic instillation during FMT provides healthy bacteria at the immediate site of injury in UC but in CD may not reach the target areas which are inflamed. Secondly, gut microbial diversity of ileal and ileocolonic CD is significantly different from healthy controls while those of colonic CD and UC were closer to each other [3]. This is consistent with host genetic and clinical studies that show that colonic and ileal CD are distinct, such that colonic CD lies between UC and ileal CD [128,129]. Perianal fistulae in CD house their own distinct dysbiotic microbiota [130].
Even within the domain of IBD, there have been small studies that have attempted to ameliorate extraintestinal manifestations or other complications of IBD. A pilot study included 10 patients with IBD (9 UC and 1 CD) with primary sclerosing cholangitis and elevated alkaline phosphatase > 1.5 times upper limit of normal, and found 3 out of 10 patients to have > 50% decline in alkaline phosphatase levels after a single session of FMT [131]. Despite the small sample size, they found that engraftment of the operational taxonomic units from donor feces was correlated with response in alkaline phosphatase levels.
Pouchitis is a frequent and bothersome complication following ileal pouch-anal anastomosis following colectomy for IBD, and interest in FMT has arisen after early studies showing dysbiosis in patients with pouchitis but not those with healthy pouch [132]. A systematic review of FMT in pouchitis identified 9 studies (predominantly case reports and series) with 69 patients and found response rates of 31% and remission rates of 22% following FMT, although with significant heterogeneity in FMT methods [133].
Response of perianal CD to FMT has not been systematically assessed even though they have been included in ongoing trials [134].
There have been numerous reports of successful treatment of CDI in patients with underlying IBD, whose mucosal immunological dysfunction and microbial dysbiosis provide a fertile ground for manifestation of CDI. A meta-analysis of 9 cohort studies found an overall cure rate of 89% for CDI, similar in patients with or without IBD, and similar success rates in CD and UC [135]. Surprisingly, 7 patients in the review required colectomy for disease flare up of IBD after FMT which was a frequent adverse event. Engraftment of microbial taxa is impaired in patients with active IBD requiring escalation of medication, although this did not result in higher recurrence of CDI on follow-up at 6 months [136]. They also found 16% and 55% prevalence of IBD flares at 2 and 6 months post-FMT.
FMT is generally considered safe, and few major side effects have been reported in trials in UC, CD, as well as children and adolescents with IBD [111,137,138]. One in 5 patients undergoing FMT experience adverse events, including gastrointestinal symptoms such as diarrhea (10%), and abdominal pain, cramps, or bloating (7%), that are considered transient, mild, and self-limiting [139]. Serious adverse events including infections or death have been reported in up to 1.4% of FMT sessions. These are of great concern since the population of patients with IBD are often on immunosuppression, have impaired gut mucosal barrier, and are malnourished. As expected, these serious adverse events are restricted to patients with mucosal barrier injury (such as ulcers or erosions) as seen in IBD, but not when used for IBS, obesity, or autism. However, the screening protocol must be meticulous -there have been reported cases of bacteremia and sepsis following FMT-such as 2 cases of drug-resistant extended spectrum beta-lactamase producing E. coli sepsis in UC (one of whom who received FMT during bone marrow suppression for hematopoietic stem cell transplantation died) [140], pneumonia and Staphylococcus epidermidis bacteremia in an 80-year-old lady with chronic obstructive pulmonary disease after oral capsule FMT for recurrent CDI [81], and E. coli bacteremia in CD with recurrent CDI [141]. Two other reported cases led to fatality but were due to technical complications- one due to difficult delivery to the duodenum leading to aspiration pneumonia [142], and the other due to sedation during colonoscopy [143]. On the other hand, a large series of 80 immunocompromised patients undergoing FMT for CDI, 36 of whom were IBD patients receiving immunosuppressants, found a single death attributed to sedation during colonoscopy and only minor self-limited adverse effects [143]. A total of 50 deaths were reported in the systematic review by Quraishi et al. [144] in patients with recurrent CDIs, most of whom the individual authors did not attribute to FMT. Four patients with a ribotype 027 strain of C. difficile did not respond to FMT and died shortly after FMT and were judged by the authors to be seriously ill prior to FMT and died as a result of CDI rather than FMT [145]. It would be prudent to conclude that the safety of FMT is not absolute, and there is a need for conscientious choice of candidate patients, meticulous screening for drug-resistant organisms, and vigilant monitoring of patients post-procedure for early identification of bacteremia and other infectious complications.
FMT has been accepted widely following revelations of its efficacy, safety, and cost-efficacy in the treatment of recurrent CDI. Authorities regulate FMT differently across countries, as depicted in Table 2 [146-151]. There remains confusion as to whether FMT constitutes a product of human tissue, or a collection of microbes alone, and is unfit for the definition of drug due to the impossibility of similarity between samples. An important milestone for regulatory approval was when the designation of FMT was downgraded from human tissue to medicinal product in the United Kingdom in 2015-which allowed greater ease of access to this therapy for a large number of patients and clinical trials across the nation [152]. FMT remains experimental in the context of use in IBD.
The FDA issued guidance statements in 2012 detailing the chemistry and manufacturing of live microbial products (LMPs), and approved the first fecal microbiota product, REBYOTA, in 2022 for preventing recurrent CDI [149,153]. LMPs include preparations containing live bacteria or viruses for therapeutic or preventative purposes, apart from vaccines. REBYOTA is a biotherapeutic product manufactured from qualified donors, with samples collected at the manufacturing site, in line with good manufacturing practices with a broad spectrum of microbes, representative of the healthy gut. It is administered rectally as 150 mL suspension within 3 days of completing antibiotic therapy and is well tolerated [154]. Success of SER-287 (spore-based microbiome therapeutic) in a phase 1b trial was followed by negative results in the phase II trial [155,156]. Further trials of preformulated LMP preparations in IBD are underway [157].
Colonoscopic transendoscopic tube insertion involves the trans-anal placement of a tube to the cecum, where it is affixed using titanium clips, for the administration of FMT [158]. The placement procedure was successful in all cases, required approximately 15 minutes, and the tube retention period was acceptable to patients in the initial observation study. The tubes remained in place for 2 weeks on average, before falling off, and during this period the patients could be administered FMT or mesalazine through this without significant discomfort.
A recent study looked at a machine learning model to predict the best donors and also the optimal engraftment rates [159]. This was based on an integrated shotgun metagenomic systematic meta-analysis of new and publicly available stool microbiomes collected from 226 triads of donors, pre-FMT recipients, and post-FMT recipients across 8 different disease types from 24 studies. This study employed new strain-resolved metagenomic approaches to elucidate the degree of FMT engraftment and associated clinical outcomes. It was seen that recipients with higher donor strain engraftment were more likely to experience clinical success after FMT. Increased engraftment was seen in individuals receiving FMT from multiple routes (e.g., both via capsules and colonoscopy during the same treatment) as well as in antibiotic-treated recipients with infectious diseases compared with antibiotic-naïve patients with noncommunicable diseases. Bacteroidetes and Actinobacteria species (including Bifidobacteria) displayed higher engraftment than Firmicutes except for 6 under-characterized Firmicutes species.
Much remains to be explored regarding FMT as a routinely available treatment option for IBD. Some gaps in our knowledge that impede this goal are listed in Table 3.
FMT is a promising treatment for IBD, but more RCTs are needed to establish its efficacy and safety. The existing trials suggest that FMT may be effective in inducing and maintaining remission in patients with UC, but its effectiveness in CD is less clear. FMT appears to be safe with few serious adverse events reported. Further research is needed to optimize the delivery of FMT, identify optimal donor selection criteria, and establish its longterm safety and effectiveness in the treatment of IBD.
ADDITIONAL INFORMATION
Fig.┬Ā1.
Overview of fecal microbiota transplantation (FMT) in inflammatory bowel disease (IBD). Fecal matter from a healthy donor is collected, processed, and administered into a patient with IBD through one of several routes, typically via colonoscopy. FMT, if engraftment is successful, modulates existing gut microbiota by promoting growth of eubionts and suppressing pathobionts, with downstream effects of promoting anti-inflammatory metabolites (such as short-chain fatty acids or secondary bile acids) and suppressing inflammatory metabolites (triacylglycerol and tetrapyrrole). The interaction with immune system is complex but generally suppresses aberrant inflammation.
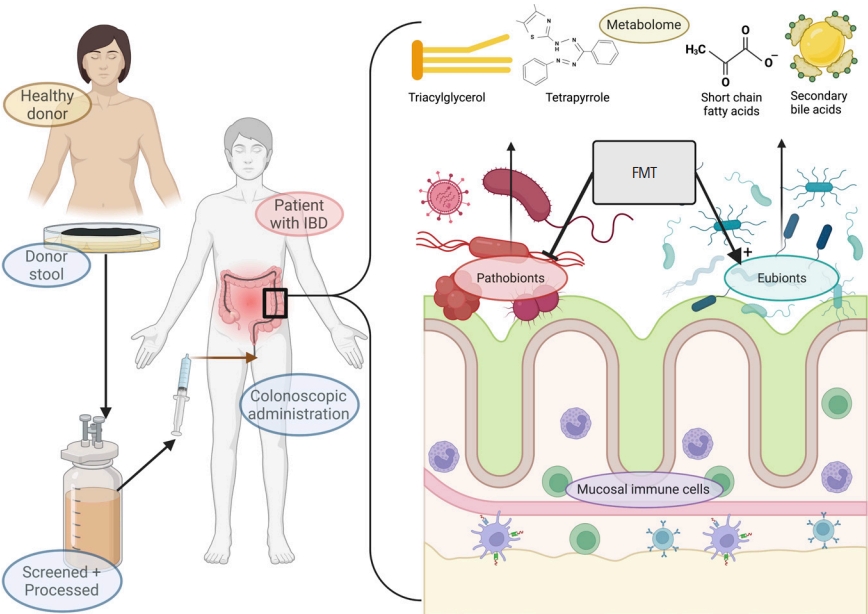
Fig.┬Ā2.
Factors that determine success of fecal microbiota transplantation (FMT) in inflammatory bowel disease (IBD). The figure shows several factors including selection of donor, FMT session frequency, choice of frozen versus fresh FMT, pretreatment with antibiotics, and different routes of administration, all of which have been found to affect success of FMT. These serve as guides to allow improvement in FMT technique and creation of an optimum protocol.

Table┬Ā1.
Clinical Trials Evaluating FMT in Patients with Ulcerative Colitis
| Study | Country | Sample sizea | Severity | Type of donor | Type of faces | Delivery route | Total dosage | Pre-antibiotics | Control | Time of evaluation | Clinical remissiona | Endoscopic remissiona | Serious AE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Induction | ||||||||||||||
| Tkach (2023) [105] | Ukraine | 26/27 | Mild to moderate active UC (partial Mayo score 4-6, with endoscopic subscore > 0) | Single donor | Fresh | Colonoscopy | 50-80 g | No | 5-ASA | 8 wk | 22/19 | 5/1 | 6/2 | |
| Kedia (2022) [91] | India | 35/31 | Mild to moderate (SCCAI 3-9) endoscopically active (UCEIS > 1) | Multiple donors (2-5) | Fresh | Colonoscopy with anti-inflammatory diet | 350 g | No | Optimization of medical therapy | 8 wk | 21/10 | 12/4 | 26/27 | |
| Haifer (2022) [80] | Australia | 15/20 | Mild to moderate (Mayo score of 4-10 and endoscopic Mayo subscore 1) | Single donor | Frozen | FMT capsules | 102.9 g | Yes | Placebo | 8 wk | 11/5 | 7/3 | 2/1 | |
| Sarbagili (2022) [106] | Israel | 19/15 | Active UC (SCCAI 5-11 and endoscopic Mayo score of 2-3) | Single donor | Frozen | Colonoscopy and retention enema | 133.3 g | No | UCED | 8 wk | 4/6 | 3/4 | 0/0 | |
| Pai (2021) [107] | Canada | 13/12 (pediatric) | Active UC | Multiple donors | Frozen | Retention enema | 600 g | No | Placebo | 30 wk | 5/4 | - | 5/1 | |
| Crothers (2021) [108] | USA | 6/6 | Mild to moderate (Mayo score of 4-10; endoscopic Mayo subscore > 1, a rectal bleeding subscore > 1, and a stool frequency subscore 1) | Multiple donors | Frozen | Colonoscopy and FMT capsules | ~62 g | Yes | Placebo | 12 wk | 2/0 | - | 1/1 | |
| Fang (2021) [109] | China | 10/10 | Active UC | Single related donor | Fresh | Colonoscopy | 50 g | No | 5-ASA | 8 wk | 9/5 | - | 1/0 | |
| Březina (2021) [110] | Czech Republic | 21/22 | Mild to moderate active left- sided UC (Mayo score of 4-10, and endoscopy subscore > 2) | Single donor | Frozen | Retention enema | 500 g | No | 5-ASA enemas | 12 wk | 12/8 | 3/3 | 4/1 | |
| Costello (2019) [60] | Australia | 38/35 | Mild to moderate (Mayo score of 3-10 and endoscopic subscore > 1) | Multiple donors (3-4) | Frozen | Colonoscopy and retention enema | 100 g | No | Autologous FMT | 8 wk | 18/6 | 4/0 | 3/2 | |
| Paramsothy (2017) [111] | Australia | 41/40 | Mild to moderate (Mayo score of 4-10) | Multiple donors (3-7) | Frozen | Colonoscopy and retention enema | 1,537.5 g | No | Placebo | 8 wk | 18/8 | 5/3 | 2/1 | |
| Rossen (2015) [79] | Netherland | 23/25 | Mild to moderate (SCCAI 4-11, and endoscopic subscore > 1) | Single donor | Fresh | Naso- duodenal infusions | ~240 g | No | Autologous FMT | 12 wk | 7/8 | 2/2 | 2/2 | |
| Moayyedi (2015) [90] | Canada | 38/37 | Mild to moderate (Mayo score of 3-10, and endoscopic subscore > = 1) | Single donor | Fresh or frozen | Retention enema | 300 g | No | Placebo | 7 wk | 9/2 | 9/2 | 3/2 | |
| Maintenance | ||||||||||||||
| Sood (2019) [112] | India | 31/30 | UC in remission (Mayo score < 3) after achieving induction of remission with FMT | Single donor | Fresh or Frozen | Colonoscopy | 700 g (100 g/session) | No | Placebo | 48 wk | 27/20 | 18/8 | 0/1 | |
| Lahtinen (2023) [113] | Finland | 24/24 | UC in remission (Mayo score < 3 and fecal calprotectin < 100 ┬Ąg/g) | Multiple donors (3) | Frozen | Colonoscopy | 30 g (single session) | No | Autologous FMT | 48 wk | 13/10 | 12/12 | 0/0 | |
Table┬Ā2.
Regulation of Fecal Microbiota Transplantation Therapy for Clostridioides difficile Infection and Inflammatory Bowel Disease across Select Countries
| Country | C. difficile infection | Inflammatory bowel disease |
|---|---|---|
| USA | Restricted use clinically in line with FDA enforcement discretion policy [148] | Experimental therapy requires investigational new drug approval or similar approval |
| Rebyota approved for clinical use [149] | ||
| Australia | Regulated as a biological drug | |
| Netherlands, Belgium, Italy | Regulated as a human tissue product, under European Union Tissue and Cells Directive (EUTCD) [150] | |
| United Kingdom, Ireland, France, Germany, Switzerland | Regulated as a medicinal drug, not tissue or biological drug-flexible use allowed [151] | |
Table┬Ā3.
Knowledge Gaps in the Practice of FMT in IBD
REFERENCES
1. Franzosa EA, Sirota-Madi A, Avila-Pacheco J, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol 2019;4:293-305.



2. Machiels K, Joossens M, Sabino J, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014;63:1275-1283.


3. Imhann F, Vich Vila A, Bonder MJ, et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 2018;67:108-119.



4. Verma R, Verma AK, Ahuja V, Paul J. Real-time analysis of mucosal flora in patients with inflammatory bowel disease in India. J Clin Microbiol 2010;48:4279-4282.




6. Nguyen LH, Ûrtqvist AK, Cao Y, et al. Antibiotic use and the development of inflammatory bowel disease: a national casecontrol study in Sweden. Lancet Gastroenterol Hepatol 2020;5:986-995.



7. Kedia S, Rampal R, Paul J, Ahuja V. Gut microbiome diversity in acute infective and chronic inflammatory gastrointestinal diseases in North India. J Gastroenterol 2016;51:660-671.



8. Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol 2011;106:563-573.



9. Naser SA, Sagramsingh SR, Naser AS, Thanigachalam S. Mycobacterium avium subspecies paratuberculosis causes CrohnŌĆÖs disease in some inflammatory bowel disease patients. World J Gastroenterol 2014;20:7403-7415.



10. Selby W, Pavli P, Crotty B, et al. Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for CrohnŌĆÖs disease. Gastroenterology 2007;132:2313-2319.


11. Khan IA, Pilli S, A S, et al. Prevalence and association of Mycobacterium avium subspecies paratuberculosis with disease course in patients with ulcero-constrictive ileocolonic disease. PLoS One 2016;11:e0152063.



12. Khan IA, Nayak B, Markandey M, et al. Differential prevalence of pathobionts and host gene polymorphisms in chronic inflammatory intestinal diseases: CrohnŌĆÖs disease and intestinal tuberculosis. PLoS One 2021;16:e0256098.



13. Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 2008;105:16731-16736.



14. Magnusson MK, Strid H, Isaksson S, Simr├®n M, ├¢hman L. The mucosal antibacterial response profile and fecal microbiota composition are linked to the disease course in patients with newly diagnosed ulcerative colitis. Inflamm Bowel Dis 2017;23:956-966.


15. Machiels K, Sabino J, Vandermosten L, et al. Specific members of the predominant gut microbiota predict pouchitis following colectomy and IPAA in UC. Gut 2017;66:79-88.


16. Zhou Y, Xu ZZ, He Y, et al. Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. mSystems 2018;3:e00188. -17.




17. Magnusson MK, Strid H, Sapnara M, et al. Anti-TNF therapy response in patients with ulcerative colitis is associated with colonic antimicrobial peptide expression and microbiota composition. J Crohns Colitis 2016;10:943-952.


18. Ananthakrishnan AN, Luo C, Yajnik V, et al. Gut microbiome function predicts response to anti-integrin biologic therapy in inflammatory bowel diseases. Cell Host Microbe 2017;21:603-610.



19. Zhou Y, He Y, Liu L, et al. Alterations in gut microbial communities across anatomical locations in inflammatory bowel diseases. Front Nutr 2021;8:615064.



20. Kim S, Kim JH, Park BO, Kwak YS. Perspectives on the therapeutic potential of short-chain fatty acid receptors. BMB Rep 2014;47:173-178.



21. Dorrestein PC, Mazmanian SK, Knight R. Finding the missing links among metabolites, microbes, and the host. Immunity 2014;40:824-832.



22. Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013;341:569-573.



23. Lamas B, Richard ML, Leducq V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 2016;22:598-605.




24. Vich Vila A, Hu S, Andreu-S├Īnchez S, et al. Faecal metabolome and its determinants in inflammatory bowel disease. Gut 2023;72:1472-1485.



25. Schoch CL, Seifert KA, Huhndorf S, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci U S A 2012;109:6241-6246.


26. Hoarau G, Mukherjee PK, Gower-Rousseau C, et al. Bacteriome and mycobiome interactions underscore microbial dysbiosis in familial CrohnŌĆÖs disease. mBio 2016;7:e01250-16. -16.




27. Hager CL, Isham N, Schrom KP, et al. Effects of a novel probiotic combination on pathogenic bacterial-fungal polymicrobial biofilms. mBio 2019;10:e00338-19.




28. Imai T, Inoue R, Kawada Y, et al. Characterization of fungal dysbiosis in Japanese patients with inflammatory bowel disease. J Gastroenterol 2019;54:149-159.



29. Jain U, Ver Heul AM, Xiong S, et al. Debaryomyces is enriched in CrohnŌĆÖs disease intestinal tissue and impairs healing in mice. Science 2021;371:1154-1159.



30. Limon JJ, Tang J, Li D, et al. Malassezia is associated with CrohnŌĆÖs disease and exacerbates colitis in mouse models. Cell Host Microbe 2019;25:377-388.



31. Stockdale SR, Shkoporov AN, Khokhlova EV, et al. Interpersonal variability of the human gut virome confounds disease signal detection in IBD. Commun Biol 2023;6:221.




32. Norman JM, Handley SA, Baldridge MT, et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 2015;160:447-460.



33. Yan A, Butcher J, Schramm L, Mack DR, Stintzi A. Multiomic spatial analysis reveals a distinct mucosa-associated virome. Gut Microbes 2023;15:2177488.



34. Markandey M, Bajaj A, Ilott NE, et al. Gut microbiota: sculptors of the intestinal stem cell niche in health and inflammatory bowel disease. Gut Microbes 2021;13:1990827.



35. Altomare A, Putignani L, Del Chierico F, et al. Gut mucosalassociated microbiota better discloses inflammatory bowel disease differential patterns than faecal microbiota. Dig Liver Dis 2019;51:648-656.


36. Kabeerdoss J, Jayakanthan P, Pugazhendhi S, Ramakrishna BS. Alterations of mucosal microbiota in the colon of patients with inflammatory bowel disease revealed by real time polymerase chain reaction amplification of 16S ribosomal ribonucleic acid. Indian J Med Res 2015;142:23-32.



37. Lennon G, Balfe Á, Bambury N, et al. Correlations between colonic crypt mucin chemotype, inflammatory grade and Desulfovibrio species in ulcerative colitis. Colorectal Dis 2014;16:-O161-O169.
38. Sokol H, Vasquez N, Hoyeau-Idrissi N, et al. Crypt abscess-associated microbiota in inflammatory bowel disease and acute self-limited colitis. World J Gastroenterol 2010;16:583587.



39. Markandey M, Bajaj A, Verma M, et al. Fecal microbiota transplantation refurbishes the crypt-associated microbiota in ulcerative colitis. iScience 2023;26:106738.



40. Abdelbary MM, Hatting M, Bott A, et al. The oral-gut axis: salivary and fecal microbiome dysbiosis in patients with inflammatory bowel disease. Front Cell Infect Microbiol 2022;12:1010853.



41. Atarashi K, Suda W, Luo C, et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 2017;358:359-365.



42. Shen ZH, Zhu CX, Quan YS, et al. Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol 2018;24:5-14.



43. Paramsothy S, Nielsen S, Kamm MA, et al. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology 2019;156:1440-1454.


44. Mocanu V, Rajaruban S, Dang J, Kung JY, Deehan EC, Madsen KL. Repeated fecal microbial transplantations and antibiotic pre-treatment are linked to improved clinical response and remission in inflammatory bowel disease: a systematic review and pooled proportion meta-analysis. J Clin Med 2021;10:959.



45. Lima SF, Gogokhia L, Viladomiu M, et al. Transferable immunoglobulin A-coated Odoribacter splanchnicus in responders to fecal microbiota transplantation for ulcerative colitis limits colonic inflammation. Gastroenterology 2022;162:166178.



46. Hoelz H, Heetmeyer J, Tsakmaklis A, et al. Is autologous fecal microbiota transfer after exclusive enteral nutrition in pediatric CrohnŌĆÖs disease patients rational and feasible? Data from a feasibility test. Nutrients 2023;15:1742.



47. Ponce-Alonso M, Garcia-Fernandez S, Aguilera L, et al. P782 A new compatibility test for donor selection for faecal microbiota transplantation in ulcerative colitis. J Crohns Colitis 2017;11(suppl_1):S480-S481.

48. U.S. Food and Drug Administration. Information pertaining to additional safety protections regarding use of fecal microbiota for transplantation: testing of stool donors for enteropathogenic Escherichia coli and Shigatoxin-producing Escherichia coli [Internet]. c2020 [cited 2023 Jul 9]. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/information-pertaining-additional-safety-protectionsregarding-use-fecal-microbiota-transplantation-0
49. Ianiro G, Mullish BH, Kelly CR, et al. Screening of faecal microbiota transplant donors during the COVID-19 outbreak: suggestions for urgent updates from an international expert panel. Lancet Gastroenterol Hepatol 2020;5:430-432.



50. B├®nard MV, de Bruijn CM, Fenneman AC, et al. Challenges and costs of donor screening for fecal microbiota transplantations. PLoS One 2022;17:e0276323.



51. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017;66:569-580.



52. Lopetuso LR, Deleu S, Godny L, et al. The first international Rome consensus conference on gut microbiota and faecal microbiota transplantation in inflammatory bowel disease. Gut 2023;72:1642-1650.



53. Bilinski J, Dziurzynski M, Grzesiowski P, et al. Fresh versus frozen stool for fecal microbiota transplantation-assessment by multimethod approach combining culturing, flow cytometry, and next-generation sequencing. Front Microbiol 2022;13:872735.



54. Gangwani MK, Aziz M, Aziz A, et al. Fresh versus frozen versus lyophilized fecal microbiota transplant for recurrent Clostridium difficile infection: a systematic review and network meta-analysis. J Clin Gastroenterol 2023;57:239-245.

55. Zhao HL, Chen SZ, Xu HM, et al. Efficacy and safety of fecal microbiota transplantation for treating patients with ulcerative colitis: a systematic review and meta-analysis. J Dig Dis 2020;21:534-548.



56. Zhang T, Lu G, Zhao Z, et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell 2020;11:251-266.




57. Ding X, Li Q, Li P, et al. Long-term safety and efficacy of fecal microbiota transplant in active ulcerative colitis. Drug Saf 2019;42:869-880.



58. Ott SJ, Waetzig GH, Rehman A, et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology 2017;152:799-811.


59. Stallmach A, Grunert P, Stallhofer J, et al. Transfer of FRozen Encapsulated multi-donor Stool filtrate for active ulcerative Colitis (FRESCO): study protocol for a prospective, multicenter, double-blind, randomized, controlled trial. Trials 2022;23:173.




60. Costello SP, Hughes PA, Waters O, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA 2019;321:156-164.



61. Shimizu H, Arai K, Asahara T, et al. Stool preparation under anaerobic conditions contributes to retention of obligate anaerobes: potential improvement for fecal microbiota transplantation. BMC Microbiol 2021;21:275.




62. Chu ND, Smith MB, Perrotta AR, Kassam Z, Alm EJ. Profiling living bacteria informs preparation of fecal microbiota transplantations. PLoS One 2017;12:e0170922.



63. Papanicolas LE, Choo JM, Wang Y, et al. Bacterial viability in faecal transplants: which bacteria survive? EBioMedicine 2019;41:509-516.



64. Singh A, Mahajan R, Kahlon BK, et al. Early fecal microbiome transfer after donor defecation determines response in patients with moderate to severe ulcerative colitis. Indian J Gastroenterol 2022;41:389-396.



65. Zhang YJ, Bousvaros A, Docktor M, et al. Higher alpha diversity and Lactobacillus blooms are associated with better engraftment after fecal microbiota transplant in inflammatory bowel disease. c2023 [cited 2023 Jul 19]. https://doi.org/10.1101/2023.01.30.23285033.


66. Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 2004;126:1620-1633.


67. Ji SK, Yan H, Jiang T, et al. Preparing the gut with antibiotics enhances gut microbiota reprogramming efficiency by promoting xenomicrobiota colonization. Front Microbiol 2017;8:1208.



68. Ishikawa D, Sasaki T, Osada T, et al. Changes in intestinal microbiota following combination therapy with fecal microbial transplantation and antibiotics for ulcerative colitis. Inflamm Bowel Dis 2017;23:116-125.


69. Kump P, Wurm P, Gr├Čchenig HP, et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment Pharmacol Ther 2018;47:67-77.




70. Mullish BH, Quraishi MN, Segal JP, et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut 2018;67:19201941.

71. Gweon TG, Lee YJ, Kim KO, et al. Clinical practice guidelines for fecal microbiota transplantation in Korea. J Neurogastroenterol Motil 2022;28:28-42.



72. Jalanka J, Salonen A, Saloj├żrvi J, et al. Effects of bowel cleansing on the intestinal microbiota. Gut 2015;64:1562-1568.


73. OŌĆÖBrien CL, Allison GE, Grimpen F, Pavli P. Impact of colonoscopy bowel preparation on intestinal microbiota. PLoS One 2013;8:e62815.



74. Harrell L, Wang Y, Antonopoulos D, et al. Standard colonic lavage alters the natural state of mucosal-associated microbiota in the human colon. PLoS One 2012;7:e32545.



75. Tariq R, Hayat M, Pardi D, Khanna S. Predictors of failure after fecal microbiota transplantation for recurrent Clostridioides difficile infection: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis 2021;40:1383-1392.



76. Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, openlabel, controlled pilot study. Clin Infect Dis 2014;58:1515-1522.



77. Yang Z, Bu C, Yuan W, et al. Fecal microbiota transplant via endoscopic delivering through small intestine and colon: no difference for CrohnŌĆÖs disease. Dig Dis Sci 2020;65:150-157.



78. Fang H, Fu L, Wang J. Protocol for fecal microbiota transplantation in inflammatory bowel disease: a systematic review and meta-analysis. Biomed Res Int 2018;2018:8941340.




79. Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015;149:110118.

80. Haifer C, Paramsothy S, Kaakoush NO, et al. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol 2022;7:141-151.


81. Kao D, Roach B, Silva M, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 2017;318:1985-1993.



82. Vaughn BP, Fischer M, Kelly CR, et al. Effectiveness and safety of colonic and capsule fecal microbiota transplantation for recurrent Clostridioides difficile infection. Clin Gastroenterol Hepatol 2023;21:1330-1337.


83. Wei ZJ, Dong HB, Ren YT, Jiang B. Efficacy and safety of fecal microbiota transplantation for the induction of remission in active ulcerative colitis: a systematic review and meta-analysis of randomized controlled trials. Ann Transl Med 2022;10:802.



84. El-Salhy M, Hatlebakk JG, Gilja OH, Br├źthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020;69:859-867.



85. Ren R, Gao X, Shi Y, et al. Long-term efficacy of low-intensity single donor fecal microbiota transplantation in ulcerative colitis and outcome-specific gut bacteria. Front Microbiol 2021;12:742255.



86. Baunwall SM, Lee MM, Eriksen MK, et al. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: an updated systematic review and meta-analysis. EClinicalMedicine 2020;29-30:100642.



87. El-Salhy M, Hausken T, Hatlebakk JG. Increasing the dose and/or repeating faecal microbiota transplantation (FMT) increases the response in patients with irritable bowel syndrome (IBS). Nutrients 2019;11:1415.



88. Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 2003;37:42-47.


89. Chen M, Liu XL, Zhang YJ, Nie YZ, Wu KC, Shi YQ. Efficacy and safety of fecal microbiota transplantation by washed preparation in patients with moderate to severely active ulcerative colitis. J Dig Dis 2020;21:621-628.




90. Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015;149:102-109.


91. Kedia S, Virmani S, K Vuyyuru S, et al. Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: a randomised controlled trial. Gut 2022;71:24012413.

92. Sood A, Singh A, Mahajan R, et al. Clinical predictors of response to faecal microbiota transplantation in patients with active ulcerative colitis. J Crohns Colitis 2020;15:238-243.


93. Kazerouni A, Wein LM. Exploring the efficacy of pooled stools in fecal microbiota transplantation for microbiota-associated chronic diseases. PLoS One 2017;12:e0163956.



94. Levast B, Fontaine M, Nancey S, Dechelotte P, Dor├® J, Lehert P. Single-donor and pooling strategies for fecal microbiota transfer product preparation in ulcerative colitis: a systematic review and meta-analysis. Clin Transl Gastroenterol 2023;14:e00568.



95. Li H, Li Y, Qian J. What is the ŌĆ£optimal formulaŌĆØ for donor selection and feces processing for fecal microbiota transplantation in ulcerative colitis? Chin Med J (Engl) 2023;136:14101412.



96. Wilson BC, Vatanen T, Cutfield WS, OŌĆÖSullivan JM. The superdonor phenomenon in fecal microbiota transplantation. Front Cell Infect Microbiol 2019;9:2.



97. Okahara K, Ishikawa D, Nomura K, et al. Matching between donors and ulcerative colitis patients is important for longterm maintenance after fecal microbiota transplantation. J Clin Med 2020;9:1650.



98. Barnes D, Ng K, Smits S, Sonnenburg J, Kassam Z, Park KT. Competitively selected donor fecal microbiota transplantation: butyrate concentration and diversity as measures of donor quality. J Pediatr Gastroenterol Nutr 2018;67:185-187.


99. Haifer C, Luu LD, Paramsothy S, Borody TJ, Leong RW, Kaakoush NO. Microbial determinants of effective donors in faecal microbiota transplantation for UC. Gut 2023;72:92-100.

100. He R, Li P, Wang J, Cui B, Zhang F, Zhao F. The interplay of gut microbiota between donors and recipients determines the efficacy of fecal microbiota transplantation. Gut Microbes 2022;14:2100197.



101. Olesen SW, Gerardin Y. Re-evaluating the evidence for faecal microbiota transplantation ŌĆśsuper-donorsŌĆÖ in inflammatory bowel disease. J Crohns Colitis 2021;15:453-461.



102. Rees NP, Shaheen W, Quince C, et al. Systematic review of donor and recipient predictive biomarkers of response to faecal microbiota transplantation in patients with ulcerative colitis. EBioMedicine 2022;81:104088.



103. Zhang B, Yang L, Ning H, et al. A matching strategy to guide donor selection for ulcerative colitis in fecal microbiota transplantation: meta-analysis and analytic hierarchy process. Microbiol Spectr 2023;11:e0215921.




104. Bajaj A, Markandey M, Singh M, et al. P702 Faecal microbiota transplantation sculpts the faecal and mucosal microbial and metabolomic profiles in patients with ulcerative colitis. J Crohns Colitis 2022;16(Suppl_1):i598-i601.


105. Tkach S, Dorofeyev A, Kuzenko I, et al. Efficacy and safety of fecal microbiota transplantation via colonoscopy as add-on therapy in patients with mild-to-moderate ulcerative colitis: a randomized clinical trial. Front Med (Lausanne) 2023;9:1049849.



106. Sarbagili Shabat C, Scaldaferri F, Zittan E, et al. Use of faecal transplantation with a novel diet for mild to moderate active ulcerative colitis: the CRAFT UC randomised controlled trial. J Crohns Colitis 2022;16:369-378.




107. Pai N, Popov J, Hill L, et al. Results of the first pilot randomized controlled trial of fecal microbiota transplant in pediatric ulcerative colitis: lessons, limitations, and future prospects. Gastroenterology 2021;161:388-393.


108. Crothers JW, Chu ND, Nguyen LT, et al. Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: results of a single-center, prospective, randomized pilot study. BMC Gastroenterol 2021;21:281.




109. Fang H, Fu L, Li X, et al. Long-term efficacy and safety of monotherapy with a single fresh fecal microbiota transplant for recurrent active ulcerative colitis: a prospective randomized pilot study. Microb Cell Fact 2021;20:18.




110. Březina J, Bajer L, Wohl P, et al. Fecal microbial transplantation versus mesalamine enema for treatment of active leftsided ulcerative colitis: results of a randomized controlled trial. J Clin Med 2021;10:2753.



111. Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 2017;389:1218-1228.


112. Sood A, Mahajan R, Singh A, et al. Role of faecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: a pilot study. J Crohns Colitis 2019;13:1311-1317.


113. Lahtinen P, Jalanka J, Mattila E, et al. Fecal microbiota transplantation for the maintenance of remission in patients with ulcerative colitis: a randomized controlled trial. World J Gastroenterol 2023;29:2666-2678.



114. El Hage Chehade N, Ghoneim S, Shah S, et al. Efficacy of fecal microbiota transplantation in the treatment of active ulcerative colitis: a systematic review and meta-analysis of double-blind randomized controlled trials. Inflamm Bowel Dis 2023;29:808-817.



115. Imdad A, Pandit NG, Zaman M, et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev 2023;4-CD012774.

116. Vuyyuru SK, Kedia S, Kalaivani M, et al. Efficacy and safety of fecal transplantation versus targeted therapies in ulcerative colitis: network meta-analysis. Future Microbiol 2021;16:12151227.

117. Haifer C, Saikal A, Paramsothy R, et al. Response to faecal microbiota transplantation in ulcerative colitis is not sustained long term following induction therapy. Gut 2021;70:22102211.

118. Cui B, Li P, Xu L, et al. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitis. J Transl Med 2015;13:298.



119. Sood A, Mahajan R, Juyal G, et al. Efficacy of fecal microbiota therapy in steroid dependent ulcerative colitis: a real world intention-to-treat analysis. Intest Res 2019;17:78-86.




120. Seth AK, Jain P. Fecal microbiota transplantation for induction of remission, maintenance and rescue in patients with corticosteroid-dependent ulcerative colitis: a long-term follow-up real-world cohort study. Intest Res 2022;20:251-259.




121. Jadhav A, Bajaj A, Xiao Y, Markandey M, Ahuja V, Kashyap PC. Role of diet-microbiome interaction in gastrointestinal disorders and strategies to modulate them with microbiome-targeted therapies. Annu Rev Nutr 2023;43:355-383.



122. Sigall-Boneh R, Levine A, Lomer M, et al. Research gaps in diet and nutrition in inflammatory bowel disease. a topical review by D-ECCO Working Group [Dietitians of ECCO]. J Crohns Colitis 2017;11:1407-1419.


123. Costello SP, Day A, Yao CK, Bryant RV. Faecal microbiota transplantation (FMT) with dietary therapy for acute severe ulcerative colitis. BMJ Case Rep 2020;13:e233135.



124. Kumagai H, Yokoyama K, Imagawa T, et al. Failure of fecal microbiota transplantation in a three-year-old child with severe refractory ulcerative colitis. Pediatr Gastroenterol Hepatol Nutr 2016;19:214-220.




125. Zhou S, Cui Y, Zhang Y, Zhao T, Cong J. Fecal microbiota transplantation for induction of remission in CrohnŌĆÖs disease: a systematic review and meta-analysis. Int J Colorectal Dis 2023;38:62.



126. ClinicalTrials.gov [Internet]. U.S. National Library of Medicine, 2022 [cited 2023 Nov 10]. https://classic.clinicaltrials.gov/ct2/show/NCT03078803
127. Sokol H, Landman C, Seksik P, et al. Fecal microbiota transplantation to maintain remission in CrohnŌĆÖs disease: a pilot randomized controlled study. Microbiome 2020;8:12.




128. Cleynen I, Boucher G, Jostins L, et al. Inherited determinants of CrohnŌĆÖs disease and ulcerative colitis phenotypes: a genetic association study. Lancet 2016;387:156-167.


129. Arora U, Kedia S, Garg P, et al. Colonic CrohnŌĆÖs disease is associated with less aggressive disease course than ileal or ileocolonic disease. Dig Dis Sci 2018;63:1592-1599.



130. Haac BE, Palmateer NC, Seaton ME, VanYPeren R, Fraser CM, Bafford AC. A distinct gut microbiota exists within CrohnŌĆÖs disease-related perianal fistulae. J Surg Res 2019;242:118-128.


131. Allegretti JR, Kassam Z, Carrellas M, et al. Fecal microbiota transplantation in patients with primary sclerosing cholangitis: a pilot clinical trial. Am J Gastroenterol 2019;114:10711079.

132. Zella GC, Hait EJ, Glavan T, et al. Distinct microbiome in pouchitis compared to healthy pouches in ulcerative colitis and familial adenomatous polyposis. Inflamm Bowel Dis 2011;17:1092-1100.


133. Cold F, Kousgaard SJ, Halkjaer SI, et al. Fecal microbiota transplantation in the treatment of chronic pouchitis: a systematic review. Microorganisms 2020;8:1433.



134. Xiang L, Ding X, Li Q, et al. Efficacy of faecal microbiota transplantation in CrohnŌĆÖs disease: a new target treatment? Microb Biotechnol 2020;13:760-769.




135. Chen T, Zhou Q, Zhang D, et al. Effect of faecal microbiota transplantation for treatment of Clostridium difficile infection in patients with inflammatory bowel disease: a systematic review and meta-analysis of cohort studies. J Crohns Colitis 2018;12:710-717.


136. Hirten RP, Grinspan A, Fu SC, et al. Microbial engraftment and efficacy of fecal microbiota transplant for Clostridium difficile in patients with and without inflammatory bowel disease. Inflamm Bowel Dis 2019;25:969-979.



137. Kunde S, Pham A, Bonczyk S, et al. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr 2013;56:597-601.


138. Suskind DL, Brittnacher MJ, Wahbeh G, et al. Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active CrohnŌĆÖs disease. Inflamm Bowel Dis 2015;21:556563.



139. Marcella C, Cui B, Kelly CR, Ianiro G, Cammarota G, Zhang F. Systematic review: the global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment Pharmacol Ther 2021;53:33-42.


140. DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med 2019;381:2043-2050.


141. Quera R, Espinoza R, Estay C, Rivera D. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with CrohnŌĆÖs disease and recurrent Clostridium difficile infection. J Crohns Colitis 2014;8:252-253.


142. Baxter M, Ahmad T, Colville A, Sheridan R. Fatal aspiration pneumonia as a complication of fecal microbiota transplant. Clin Infect Dis 2015;61:136-137.


143. Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol 2014;109:10651071.
144. Quraishi MN, Widlak M, Bhala N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther 2017;46:479-493.



145. Mattila E, Uusitalo-Sepp├żl├ż R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology 2012;142:490-496.


146. Merrick B, Allen L, Zain NMM, Forbes B, Shawcross DL, Goldenberg SD. Regulation, risk and safety of faecal microbiota transplant. Infect Prev Pract 2020;2:100069.



147. Porcari S, Benech N, Valles-Colomer M, et al. Key determinants of success in fecal microbiota transplantation: from microbiome to clinic. Cell Host Microbe 2023;31:712-733.


148. U.S. Food and Drug Administration. Enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies [Internet]. c2022 [cited 2023 Jul 9]. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcementpolicy-regarding-investigational-new-drug-requirementsuse-fecal-microbiota
149. U.S. Food and Drug Administration. FDA approves first fecal microbiota product [Internet]. c2022 [cited 2023 May 8]. https://www.fda.gov/news-events/press-announcements/fda-approves-first-fecal-microbiota-product
150. European Commission. Proposal for a Regulation on substances of human origin [Internet]. c2023 [cited 2023 Jul 10]. https://health.ec.europa.eu/blood-tissues-cells-and-organs/overview/proposal-regulation-substances-human-origin_en
151. de Stefano MC, Mazzanti B, Vespasiano F, Lombardini L, Cardillo M. The regulatory approach for faecal microbiota transplantation as treatment for Clostridioides difficile infection in Italy. Antibiotics (Basel) 2022;11:480.



152. Medicines and Healthcare products Regulatory Agency. A guide to what is a medicinal product [Internet]. c2020 [cited 2023 Jul 10]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/872742/GN8_FINAL_10_03_2020__combined_.pdf
153. U.S. Food and Drug Administration Center for Biologics Evaluation and Research. Early clinical trials with live biotherapeutic products: chemistry, manufacturing, and control information [Internet]. c2021 [cited 2023 Sep 9]. https://www.fda.gov/media/82945/download
154. Lee C, Louie T, Bancke L, et al. Safety of fecal microbiota, livejslm (REBYOTAŌäó) in individuals with recurrent Clostridioides difficile infection: data from five prospective clinical trials. Therap Adv Gastroenterol 2023;16:17562848231174277.




155. Henn MR, OŌĆÖBrien EJ, Diao L, et al. A phase 1b safety study of SER-287, a spore-based microbiome therapeutic, for active mild to moderate ulcerative colitis. Gastroenterology 2021;160:115-127.


156. Businesswire. Seres therapeutics announces topline results for SER-287 phase 2b study in mild-to-moderate ulcerative colitis [Internet]. c2021 [cited 2023 Jul 18]. https://www.businesswire.com/news/home/20210722005276/en/Seres-Therapeutics-Announces-Topline-Results-for-SER-287-Phase-2bStudy-in-Mild-to-Moderate-Ulcerative-Colitis
157. Oka A, Sartor RB. Microbial-based and microbial-targeted therapies for inflammatory bowel diseases. Dig Dis Sci 2020;65:757-788.












