|
 |
- Search
| Intest Res > Volume 12(2); 2014 > Article |
|
Abstract
Colonoscopy is currently regarded as the gold standard and preferred screening method for colorectal cancer (CRC). Recently, however, a limitation of colonoscopy in the prevention of CRCs has been identified, particularly in the right-sided colon, and the problem of so-called interval cancers has emerged. The prevalence of interval cancer is estimated to be between 4% and 8% of CRCs detected. Although the exact etiology of interval cancer remains unknown, factors implicated in the development of interval cancers include missed lesions at the time of colonoscopy, incomplete resection of previous neoplastic lesions, different tumor biology, and serrated pathway of carcinogenesis. However, recent evidence suggests that interval cancers are related to the training of the endoscopist and quality of the colonoscopy rather than tumor biology. Therefore, the importance of adequate training and continuous monitoring of the colonoscopy quality, which are amenable to improvement, cannot be overstated in order to prevent the risk of interval cancers. In this study, the current literature regarding the prevalence and potential factors related to interval cancers and colonoscopy quality-related issues are reviewed.
The incidence of colorectal cancer (CRC) in Korea has been markedly increasing in recent years. This epidemiologic change in Korea requires an increasing number of colonoscopies, which are highly effective in reducing the incidence and mortality of CRC.1,2,3 Colonoscopy is currently regarded as the gold standard and preferred screening method for CRC. However, evidence suggests that colonoscopy is not as sensitive for the detection of neoplasia as previously believed, as demonstrated in studies analyzing the effectiveness of tandem colonoscopy and CT colonography.4,5 A systemic review of six studies on tandem colonoscopy showed that the pooled miss rate for polyps of any size was 22%.4 Using CT colonography as a reference standard, the miss rate of conventional colonoscopy for a large adenoma (≥10 mm) was 12%.5 As a result, a significant number of colorectal neoplasias, including cancers, may be missed, even by experienced endoscopists. Moreover, results from the National Polyp Study and several other studies6,7,8 suggested that colonoscopy is associated with only a 37-65% reduction in mortality from CRC, which is much lower than that reported previously. This could be attributed to the limited effectiveness of colonoscopic prevention of CRC, especially in the right-sided colon. Therefore, the role and limitations of colonoscopic screening must be carefully reconsidered.
The problem of the so-called interval cancers or missed cancers has been highlighted recently.9,10,11,12 CRCs detected in patients who have received colonoscopies within the surveillance interval are called interval cancers, and if they arise from missed lesions, they are also called missed cancers.13 This raises a question regarding the precise prevalence of interval cancer. Early studies have shown that 4-5% of CRCs may be missed on a single colonoscopic examination. 9,10 However, recent studies reported an increase in the prevalence of interval cancers of up to 8%.11,12
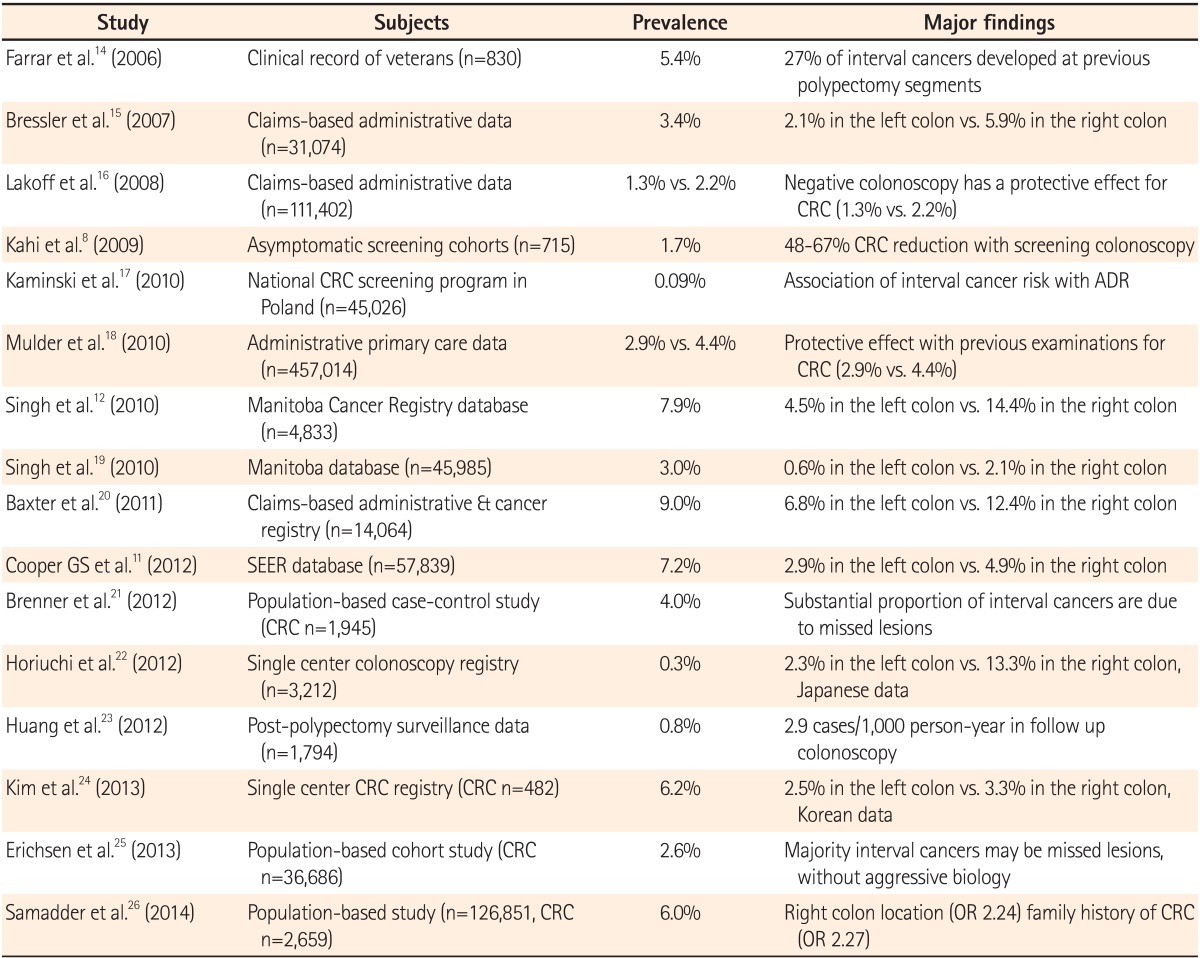
Table 1 summarizes the major findings of studies conducted since 2006 reflecting the prevalence of interval cancers.8,11,12,14,15,16,17,18,19,20,21,22,23,24,25,26 Based on 57,839 patients from the Surveillance, Epidemiology, and End Results-Medicare database, 7.2% of patients developed interval cancers.11 In a Manitoba Cancer Registry database, approximately 7.9% (approximately 1 in 13) of 4,833 CRCs were classified as missed cancers.12 In Korea, the prevalence of interval cancer was reported to be 6.2% by Kim et al.24; however, this study was limited by referral bias, selection bias, and recall bias, as it was based on data obtained by telephone calls from a single tertiary referral center. Therefore, well-designed population studies are necessary to determine the prevalence of interval cancer in Korea. In a population-based study from a Danish group,25 interval cancer (colonoscopies performed 1-5 years before diagnosis) was 2.6% among 38,064 CRC cases diagnosed between 2000 and 2009. In a population-based study conducted in Utah,26 6% of all patients with CRC had interval cancers (colonoscopies performed 6-60 months before diagnosis) among 2,659 CRCs diagnosed between 1995 and 2009. Most previous studies on interval cancers may be limited, as they relied on registry or administrative data, which frequently lack detail and might be less appropriate for the identification of underlying causes. Previous studies were also limited by the fact that definitions of interval cancer were restricted to the initial 3-5 years after colonoscopy and did not specifically focus on a complete negative colonoscopy (i.e., a colonoscopy with no detection of adenoma). To overcome these limitations, a population-based study was performed in Germany using the definition of interval cancer as a CRC occurring within 10 years after a complete negative colonoscopy.21 Using this 10-year definition, 4.0% of interval cancers were identified among 1,945 CRC cases diagnosed between 2003 and 2007.
To interpret the large variation in the prevalence of interval cancer, several factors should be considered: differences in the study design (retrospective vs. prospective), the different definitions of interval cancers (3-5 years vs. 10 years), the use of data (claims-based administrative vs. clinical data), differences in the study population (screening vs. diagnostic indications), and differences in endoscopist specialty.27 However, most studies have shown that colonoscopy offers suboptimal protection against CRC, especially in the right colon. We could estimate the magnitude of this risk in our routine practice in Korea. Assuming that proximately 20,588 new cases of CRC were diagnosed (data in 2007) based on the Korea National Cancer Incidence Database28,29 and considering an average risk scenario (e.g., 4-8% of diagnosed CRCs are interval cancers), approximately 823-1,647 interval cancers might be expected to occur annually in Korea. Considering the increasing age of the general population and the number of colonoscopies performed in Korea, the incidence of interval cancer is expected to increase. Regardless of the magnitude of this problem, the medical and social impact of the failure of colonoscopy to prevent CRCs may be devastating.27 Therefore, prompt elucidation of the factors implicated in interval cancers is necessary.
Although the exact etiology of interval cancer is unknown, there are several potential explanations for such interval cancers.
First, precancerous or cancerous lesions may have been missed at the time of colonoscopy.30 Withdrawal time and technique deserve special consideration in this context, as the detection rate of colorectal neoplasia depends on the withdrawal time during colonoscopy. Barclay et al.31 demonstrated that a withdrawal time ≥6 min is strongly correlated with an increased adenoma detection rate (ADR). In addition, careful inspection of the oral side of the mucosal folds is more important than simple adherence to a standard withdrawal time, especially in the proximal colon.32,33 The ADR itself is also an important quality indicator for predicting the risk of interval cancers after screening colonoscopy. Kaminski et al.17 demonstrated that the ADR of endoscopist was significantly associated with the risk of interval cancer (P=0.008). Endoscopies with an ADR of less than 11.0% were associated with a 10.94-fold (95% CI, 1.37-87.01) higher risk of interval cancer than endoscopies with an ADR of 20.0% or higher. Complete colonoscopy, another quality indicator of colonoscopy, is also associated with the risk of interval cancer.9 In a previous study conducted in New Zealand, 9 of 17 interval cancers occurred after an incomplete colonoscopy.34 Brenner et al.21 also showed that 18% of interval cancers were associated with the completeness of the preceding negative colonoscopy. In this study, interestingly, incompleteness of colonoscopy was associated with distal interval cancers as well as proximal interval cancers, which was explained by a strong association between incompleteness and other aspects of colonoscopy quality, including the miss rate in the distal colon. Non-polypoid lesions, which have a subtle endoscopic appearance, may be more easily missed, especially by endoscopists with lower ADR and in inadequate bowel preparations.13 Previous several studies showed that non-polypoid lesions and interval cancers share some common features:15,19,35,36 both lesions are common in the proximal colon,15,19,35 and interval cancers have a flat appearance.36 Although the relationship between non-polypoid lesions and interval cancers has not yet been clarified, endoscopists should be careful not to miss non-polypoid lesions during colonoscopy, especially in the right-sided colon.
Second, interval cancers may develop from the incomplete resection of previous neoplastic lesions.37,38,39 The recent complete adenoma resection study showed that complete resection of neoplasia is far less common than previously thought.37 In the complete adenoma resection study, the incomplete resection rate varied broadly (6.5-22.7%) among endoscopists, and was significantly higher in large and sessile lesions. Robertson et al.38 showed that 26% of interval cancers occur in the same anatomic segments as those of a previous polypectomy, suggesting a possible role for incomplete polypectomy in the development of interval cancers. A dietary polyp prevention trial showed a similar rate of interval cancers after incomplete polypectomy.36 In this trial, in 4 of 13 patients, interval cancers were associated with incomplete polypectomy. Lieberman et al.39 showed that the 5-year interval cancer rate after a normal colonoscopy without polypectomy is 0.17%, whereas the 5-year interval cancer rate after a previous polypectomy is 1.5%. These findings suggest that interval cancers that develop from lesions are visualized and recognized but are incompletely removed during a prior colonoscopic polypectomy.
Third, different biological factors associated with molecular carcinogenesis may contribute to the development of interval cancers, especially in the proximal colon.40,41 Arain et al.40 showed that interval cancers are more likely to arise in the right colon and have microsatellite instability (MSI) and CpG island methylator phenotypes (CIMP) than non-interval cancers. In multivariable logistic regression model, MSI (OR: 2.7; 95% CI: 1.1-6.8) and CIMP (OR: 2.41; 95% CI: 1.2-4.9) were independently associated with interval cancers. Sawhney et al.41 showed that interval cancers were 3.7 times more likely to show MSI than non-interval cancers, and Shaukat et al.42 showed that BRAF mutation is not associated with interval cancers. However, these three studies were limited as they were conducted by the same author group using the same samples. Therefore, additional studies on the molecular characteristics of interval cancers are warranted.
Finally, the serrated pathway for carcinogenesis of CRC has recently been the focus of studies, as sessile, serrated adenomas (SSAs) have a malignant potential that is at least as high or higher than the malignant potential of conventional adenomas.43,44 SSAs are usually difficult to detect by colonoscopy, as they are often located in the right colon with a sessile configuration and an inconspicuous border. Colonoscopists often face difficulties in recognizing and completely removing SSAs, and as a result, a subset of these lesions potentially progress to interval cancers. SSAs share common features with interval cancers, such as right colon predominance, MSI, and a CIMP-high phenotype.44 In a multicenter study involving 32 endoscopy centers,45 the detection of proximal serrated lesions varied greatly among endoscopy centers (P<0.0001), and there was also substantial variation among pathologists in the identification of sessile, serrated lesions. Fortunately, clinically significantly serrated polyps may be easier to detect with longer withdrawal times.46 The New Hampshire Colonoscopy Registry study showed that a withdrawal time of 9 min resulted in a nearly 30% relative increase in serrated polyp detection.46 Therefore, systemic education programs on serrated lesions as well as colonoscopy quality may improve the recognition and diagnosis of serrated lesions.
Recent evidence suggests that interval cancers are caused by a deficiency in the quality of colonoscopy rather than accelerated tumor biology.12,19 This is good news, as most interval cancers may be prevented by improving colonoscopy quality.
First, as described above, interval cancers may develop from suboptimal quality indicators such as withdrawal time, ADR, complete colonoscopy, and bowel preparation, as well as incomplete resection of previous neoplastic lesions.37,38,39 In addition, the identification of serrated lesions may be improved with longer withdrawal time.46
Second, the grade, stage, histology, and survival patterns do not differ between patients with interval cancer and those with non-interval cancer, which does not suggest an aggressive biology, but rather that the majority represent missed lesions.25,47 In a Danish population-based registry,25 982 Danish individuals with interval cancers were compared to two reference groups, namely, 358 individuals with CRC identified more than 10 years after a colonoscopy and 35,707 individuals with CRC but no prior colonoscopy. In this study, no significant differences were found in the characteristics of patients and tumors or survival between interval cancers and cancers arising more than 10 years after colonoscopy.25 The adjusted mortality rate ratios of interval cancers to non-interval cancers were 0.92 and 1.0 after 1 years and 5 years, respectively.25
Finally, cases of missed cancer are more common when the colonoscopy is performed by non-gastroenterologists.12,48 A recent Canadian registry study12 showed that endoscopist specialty remains a significant predictor of missed cancers despite adjustment for procedural volume. Rabeneck et al.48 also showed that non-gastroenterologists have a significantly higher rate of missed cancers than gastroenterologists, independent of procedural volume. The lack of an association between colonoscopy volume and missed cancers may indicate that even non-gastroenterologists who handle a high volume of procedures continue to miss more CRCs than gastroenterologists. It may also indicate that formal endoscopic training generally leads to competency in colonoscopy, whereas providers who do not receive formal training are unable to achieve competence despite the volume of procedures they perform.12,47,48 A higher rate of interval cancers associated with index colonoscopies performed by general practice physicians was also found in studies from Ontario and Indiana.15,49 Emerging evidence suggests a link between endoscopist specialty and colonoscopy quality in Korea.50 To the best of our knowledge, there is no formal curriculum or guidelines in colonoscopy training for general practice physicians in Korea. Therefore, more stringent standards for training and assessment of colonoscopy quality are necessary to cope with the risk of interval cancer.
Interval cancers may lead to medicolegal action against colonoscopists. Generally, plaintiffs often allege inadequate performance of colonoscopy as the proximate cause of the interval cancer. To date, there is no published data on malpractice claims related with interval cancer. A review of malpractice claims filed with the Physicians Insurers Association of America (database queried between 1985 and 2008) determined that colonoscopy represents the highest frequency (41.5%) of closed claims and the highest total indemnities ($ 54,093,000) among gastrointestinal endoscopy claims.51 Indeed, there was an average increase of 15.5% per year in total claim payments associated with colonoscopy, and the majority resulted from inadequate performance of an endoscopic procedure, followed by diagnosis error (may include cases of interval cancer). In Korea, a review of malpractice claims reported from the Korea Consumer Agency (database queried between 2009 and 2011) determined an increase of 205% in total claim consultations.52 In addition, the rate of diagnostic error for CRCs was 6.8% of all cases of cancers associated with diagnostic errors. These data do not provide detailed information about interval cancer; however, out anecdotal impression is that lawsuits alleging missed cancers are increasing. Given the increased use of colonoscopy, the number of medicolegal litigation related to interval cancers would be logically expected to increase.
Colonoscopists are encouraged to reduce their risk by documenting the limitations of colonoscopy in the informed consent process and their data of colonoscopy quality (e.g., cecal intubation, bowel preparation, and withdrawal time). Colonoscopists commonly discuss the complications of the procedure; however, they seldom discuss the possibility of missed lesions and failure to prevent interval cancers.16 Colonoscopy is not considered a perfect method by colonoscopists or by patients. Informed consent may shift the burden of this reality from the colonoscopists to the patients. Maintenance of an adequate level of colonoscopy quality indicators and detection of non-polypoid lesions, including SSAs, especially in the right colon, may minimize the risk of medicolegal litigation related with interval cancer.
Considering the significant number of interval cancers that are encountered in daily clinical practice, the importance of adequate training and improvement of colonoscopy quality as causative factors in interval cancers should be highlighted. Continuous monitoring of colonoscopy quality, which is amenable to improvement, cannot be overstated to prevent the occurrence of interval cancers.
References
1. Winawer SJ, Zauber AG, Ho MN, et al. The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med 1993;329:1977-1981.PMID: 8247072.


2. Jorgensen OD, Kronborg O, Fenger C. The Funen Adenoma Follow-up Study. Incidence and death from colorectal carcinoma in an adenoma surveillance program. Scand J Gastroenterol 1993;28:869-874.PMID: 8266015.


3. Thiis-Evensen E, Hoff GS, Sauar J, Langmark F, Majak BM, Vatn MH. Population-based surveillance by colonoscopy: effect on the incidence of colorectal cancer. Telemark Polyp Study I. Scand J Gastroenterol 1999;34:414-420.PMID: 10365903.


4. van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 2006;101:343-350.PMID: 16454841.


5. Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Ann Intern Med 2004;141:352-359.PMID: 15353426.


6. Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687-696.PMID: 22356322.



7. Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009;150:1-8.PMID: 19075198.


8. Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol 2009;7:770-775.PMID: 19268269.


9. Haseman JH, Lemmel GT, Rahmani EY, Rex DK. Failure of colonoscopy to detect colorectal cancer: evaluation of 47 cases in 20 hospitals. Gastrointest Endosc 1997;45:451-455.PMID: 9199899.


10. Gorski TF, Rosen L, Riether R, Stasik J, Khubchandani I. Colorectal cancer after surveillance colonoscopy: false-negative examination or fast growth? Dis Colon Rectum 1999;42:877-880.PMID: 10411433.


11. Cooper GS, Xu F, Barnholtz Sloan JS, Schluchter MD, Koroukian SM. Prevalence and predictors of interval colorectal cancers in medicare beneficiaries. Cancer 2012;118:3044-3052.PMID: 21989586.


12. Singh H, Nugent Z, Demers AA, Bernstein CN. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol 2010;105:2588-2596.PMID: 20877348.



14. Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol 2006;4:1259-1264.PMID: 16996804.


15. Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology 2007;132:96-102.PMID: 17241863.


16. Lakoff J, Paszat LF, Saskin R, Rabeneck L. Risk of developing proximal versus distal colorectal cancer after a negative colonoscopy: a population-based study. Clin Gastroenterol Hepatol 2008;6:1117-1121.PMID: 18691942.


17. Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010;362:1795-1803.PMID: 20463339.


18. Mulder SA, van Soest EM, Dieleman JP, et al. Exposure to colorectal examinations before a colorectal cancer diagnosis: a case-control study. Eur J Gastroenterol Hepatol 2010;22:437-443.PMID: 19952765.


19. Singh H, Nugent Z, Mahmud SM, Demers AA, Bernstein CN. Predictors of colorectal cancer after negative colonoscopy: a population-based study. Am J Gastroenterol 2010;105:663-673.PMID: 19904239.



20. Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology 2011;140:65-72.PMID: 20854818.


21. Brenner H, Chang-Claude J, Seiler CM, Hoffmeister M. Interval cancers after negative colonoscopy: population-based casecontrol study. Gut 2012;61:1576-1582.PMID: 22200840.


22. Horiuchi A, Nakayama Y, Kajiyama M, Kamijima T, Tanaka N. Invasive colorectal cancer within 5 years of negative colonoscopy in a Japanese population. Colorectal Dis 2012;14:1090-1094.PMID: 22107065.


23. Huang Y, Gong W, Su B, Zhi F, Liu S, Jiang B. Risk and cause of interval colorectal cancer after colonoscopic polypectomy. Digestion 2012;86:148-154.PMID: 22889865.


24. Kim CJ, Jung YS, Park JH, et al. Prevalence, clinicopathologic characteristics, and predictors of interval colorectal cancers in Korean population. Intest Res 2013;11:178-183.

25. Erichsen R, Baron JA, Stoffel EM, Laurberg S, Sandler RS, Sorensen HT. Characteristics and survival of interval and sporadic colorectal cancer patients: a nationwide population-based cohort study. Am J Gastroenterol 2013;108:1332-1340.PMID: 23774154.



26. Samadder NJ, Curtin K, Tuohy TM, et al. Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology doi:10.1053/j.gastro.2014.01.013. Published online ahead of print 11 Jan 2014.

27. Sanduleanu S, Masclee AM, Meijer GA. Interval cancers after colonoscopy-insights and recommendations. Nat Rev Gastroenterol Hepatol 2012;9:550-554.PMID: 22907162.



28. Shin A, Kim KZ, Jung KW, et al. Increasing trend of colorectal cancer incidence in Korea, 1999-2009. Cancer Res Treat 2012;44:219-226.PMID: 23341785.




29. Park HC, Shin A, Kim BW, et al. Data on the characteristics and the survival of korean patients with colorectal cancer from the Korea central cancer registry. Ann Coloproctol 2013;29:144-149.PMID: 24032114.



30. Pohl H, Robertson DJ. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol 2010;8:858-864.PMID: 20655393.


31. Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006;355:2533-2541.PMID: 17167136.


32. Lee RH, Tang RS, Muthusamy VR, et al. Quality of colonoscopy withdrawal technique and variability in adenoma detection rates (with videos). Gastrointest Endosc 2011;74:128-134.PMID: 21531410.


33. Rex DK. Looking over your shoulder during colonoscopy: potential roles for videorecording colonoscopy withdrawals. Gastrointest Endosc 2012;75:134-137.PMID: 22196812.


34. Leaper M, Johnston MJ, Barclay M, Dobbs BR, Frizelle FA. Reasons for failure to diagnose colorectal carcinoma at colonoscopy. Endoscopy 2004;36:499-503.PMID: 15202045.



35. Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA 2008;299:1027-1035.PMID: 18319413.


36. Pabby A, Schoen RE, Weissfeld JL, et al. Analysis of colorectal cancer occurrence during surveillance colonoscopy in the dietary Polyp Prevention Trial. Gastrointest Endosc 2005;61:385-391.PMID: 15758908.


37. Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology 2013;144:74-80.PMID: 23022496.


38. Robertson DJ, Greenberg ER, Beach M, et al. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology 2005;129:34-41.PMID: 16012932.


39. Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology 2007;133:1077-1085.PMID: 17698067.


40. Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol 2010;105:1189-1195.PMID: 20010923.



41. Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology 2006;131:1700-1705.PMID: 17087932.


42. Shaukat A, Arain M, Thaygarajan B, Bond JH, Sawhney M. Is BRAF mutation associated with interval colorectal cancers. Dig Dis Sci 2010;55:2352-2356.PMID: 20300843.


43. Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 2006;131:1400-1407.PMID: 17101316.


44. Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol 2011;42:1-10.PMID: 20869746.


45. Payne SR, Church TR, Wandell M, et al. Endoscopic detection of proximal serrated lesions and pathologic identification of sessile serrated adenomas/polyps vary on the basis of center. Clin Gastroenterol Hepatol doi:10.1016/j.cgh.2013.11.034. Published online ahead of print 10 Dec 2013.

46. Butterly L, Robinson CM, Anderson JC, et al. Serrated and adenomatous polyp detection increases with longer withdrawal time: results from the new hampshire colonoscopy registry. Am J Gastroenterol 2014;109:417-426.PMID: 24394752.




47. Inadomi J. Interval cancers after colonoscopy: the importance of training. Am J Gastroenterol 2010;105:2597-2598.PMID: 21131928.



48. Rabeneck L, Paszat LF, Saskin R. Endoscopist specialty is associated with incident colorectal cancer after a negative colonoscopy. Clin Gastroenterol Hepatol 2010;8:275-279.PMID: 19879970.


49. Rex DK, Rahmani EY, Haseman JH, Lemmel GT, Kaster S, Buckley JS. Relative sensitivity of colonoscopy and barium enema for detection of colorectal cancer in clinical practice. Gastroenterology 1997;112:17-23.PMID: 8978337.


50. Cha JM, Han DS, Lee HL, et al. Endoscopist specialty is associated with high-quality endoscopy in Korea. Yonsei Med J 2012;53:310-317.PMID: 22318818.



51. Hernandez LV, Klyve D, Regenbogen SE. Malpractice claims for endoscopy. World J Gastrointest Endosc 2013;5:169-173.PMID: 23596540.



52. Second bureau of damage relief: Medical team [Report]. Research on the actual condition of consumer damage related to the diagnosis error of cancer (Korea).. Seoul: Korea Consumer Agency, 2012:1-11.