 |
 |
- Search
| Intest Res > Volume 15(2); 2017 > Article |
|
Abstract
Background/Aims
Parthenolide (PT), a principle component derived from feverfew (Tanacetum parthenium), is a promising anticancer agent and has been shown to promote apoptotic cell death in various cancer cells. In this study, we focused on its functional role in apoptosis, migration, and invasion of human colorectal cancer (CRC) cells.
Methods
SW620 cells were employed as representative human CRC cells. We performed the MTT assay and cell cycle analysis to measure apoptotic cell death. The wound healing, Transwell migration, and Matrigel invasion assays were performed to investigate the effect of PT on cell migration/invasion. Western blotting was used to establish the signaling pathway of apoptosis and cell migration/invasion.
Results
PT exerts antiproliferative effect and induces apoptotic cell death of SW620 cells. In addition, PT prevents cell migration and invasion in a dose-dependent manner. Moreover, PT markedly suppressed migration/invasion-related protein expression, including E-cadherin, β-catenin, vimentin, Snail, cyclooxygenase-2, matrix metalloproteinase-2 (MMP-2), and MMP-9 in SW620 cells. PT also inhibited the expression of antiapoptotic proteins (Bcl-2 and Bcl-xL) and activated apoptosis terminal factor (caspase-3) in a dose-dependent manner.
Colorectal cancer (CRC) is one of the most prevalent cancer types worldwide. It is the third and second most diagnosed cancer in males and females, respectively, with over 1.2 million new cases and more than 6 million deaths reported annually.1 In recent years, although incidence rates have varied in different regions, a reduction in CRC mortality rates has been observed in several countries worldwide. This is most likely attributable to CRC screening, reduced prevalence of risk factors, and/or improved therapies.2,3 Currently, surgery is the most common treatment for early stage CRC, while chemotherapies are customarily administered following surgery in late stage CRC. However, the clinical prognoses of advanced CRC patients are still not optimistic.4,5 Thus, it is important to clarify the molecular mechanisms underlying CRC progression to develop new therapies that inhibit CRC progression.
Parthenolide (PT), isolated from extracts of Mexican-Indian medicinal plants has been shown to exhibit anti-inflammatory properties, and it has been used for the clinical treatment of migraines.6,7 The biological activity of PT is related to its ability to inhibit nuclear factor-κB signaling.6,8,9,10 Recent studies have demonstrated that PT exhibits anticancer property by inducing apoptosis in a number of human cancer cells.11,12 We have also demonstrated that PT induces apoptotic cell death via promoting mitochondrial dysfunction. Further, it suppressed CRC growth in xenograft models.13,14
Tumor cells possess the ability to migrate from the original site to the blood/lymph, thereby invading surrounding or distance tissue and causing metastasis. Multiple pathways and abnormal expression of molecules contribute to cancer metastasis.15 Therefore, it is important to determine the regulatory mechanisms underlying metastasis in cancer. Epithelial-mesenchymal transition (EMT) is an essential mechanism in the progression and metastasis of cancer.16 EMT facilitates the metastasis of epithelium-derived tumor cells by promoting the epithelial cells to loss their cell-cell adhesion and obtaining migratory and invasive properties. Some studies have evaluated the effect of PT on cell migration and invasion related to apoptosis in pancreatic and ovarian cancer cells.17,18 However, whether PT affects the migration and invasion of human CRC cells remains unclear. Therefore, in the present study, we evaluated the effect of PT on the apoptosis and migration/invasion of human SW620 CRC cells and determined the possible signaling pathways.
PT was obtained from Calbiochem (San Diego, CA, USA), and it was dissolved in dimethylsulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) to obtain a concentration of 100 µM and then stored at −20℃ in the dark. Growth factorreduced Matrigel was purchased from BD Biosciences (San Diego, CA, USA). Anticyclooxygenase-2 (anti-COX-2), anti-Bcl-2, anti-Bcl-xL, and anticaspase-3 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-E-cadherin, anti-β-catenin, antivimentin, antimatrix metalloproteinase-9 (anti-MMP-9), and anti-cleaved caspase-3 were from Cell Signaling Technology (Danvers, MA, USA). Anti-MMP-2 and anti-Snail were from Abcam (Cambridge, UK). Antiactin was purchased from Sigma-Aldrich.
CRC cell line SW620 was obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in RPMI-1640 medium (GIBCO-BRL; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; GIBCO-BRL), 100 µg/mL penicillin (GIBCO-BRL), and 100 µg/mL streptomycin (GIBCO-BRL), followed by incubation in a humidified incubator at 37℃ under 5% CO2. For PT treatment, cells were sub-cultured in media without FBS for 24 hours. PT was diluted with FBS-free media to achieve the desired concentrations.
SW620 cells were plated at a density of 5×103 cells per well in 96-well plates and treated with PT for 24 hours. Thereafter, the media were removed from each well and replaced with 200 µL of fresh media and 20 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 2.5 mg dissolved in 50 µL of DMSO). After 3 hours of incubation at 37℃, the culture medium containing MTT was removed and 200 µL of DMSO was added. Plates were placed on a plate shaker until the crystals were dissolved. Viability was detected by measuring the absorbance at 570 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Distribution of cells in different phases of the cell cycle including sub-G1 was determined by staining DNA with propidium iodide (Sigma-Aldrich) (excitation/emission, 488/617 nm). Briefly, 1×106 cells were incubated with various concentration of PT for 24 hours. Thereafter, cells were washed with cold 1× phosphate-buffered saline (PBS) and fixed in 70% ethanol overnight. The cells were washed again with 1× PBS, incubated with propidium iodide (10 µg/mL), and simultaneously treated with RNase at 37℃ for 1 hour. The percentages of cells in the different phases of the cell cycle were measured using a BD LSR flow cytometer and analyzed using the CellQuest software (Becton Dickinson, New York, NY, USA).
SW620 cells were seeded in 6-well dishes at a density of 1×106 per well and treated with various concentration of PT. After 24 hours of incubation, a scratch in the cell monolayer was made using a sterile micropipette tip. Cells were washed twice with fresh media, and images were captured using an inverted microscope (IX71; Olympus, Center Valley, PA, USA) at 24 hours after scratching. The rate of wound healing was estimated by measuring the distance between the borders of the wound.
Migration was analyzed by using a modified Transwell chamber assay using cell culture inserts with a polycarbonate filter (24-wells, 8-µm pore size with polycarbonate membrane; SPL Life Sciences, Pocheon, Korea). Cells were pretreated with various concentration of PT for 24 hours.
Invasion assays were performed by using the same Transwell chamber with growth factor-reduced Matrigel. Briefly, 1×105 cells per well were seeded onto Matrigel-coated inserts and allowed to invade for 48 hours. Cells remaining above the insert membrane were removed with a cotton swab, and cells that invaded through the Matrigel were fixed in 25% methanol. After washing in cold 1× PBS, the cells were stained with 0.1% crystal violet in 25% methanol. The inserts were washed three times in 1× PBS and air-dried. The numbers of invaded cells on the representative sections were counted using an inverted microscope (IX71; Olympus) at 10× magnification. Five fields were counted per filter in each group; the number of invaded cells for each sample represents the average of triplicate wells over three experiments.
Treated cells were lysed on ice in RIPA buffer (Thermo Fisher Scientific, Seoul, Korea) with proteinase inhibitors (GenDEPOT, Barker, TX, USA). The protein concentration in cell lysates was measured using a Protein Quantification kit from Bio-Rad (Hercules, CA, USA). Fifty micrograms of protein per lane was loaded onto an sodium dodecyl sulfatepolyacrylamide gel. After transferring and blocking, the polyvinylidene difluoride membrane was probed with various antibodies. The binding of antibody to antigen was detected using enhanced ECL prime (GE Healthcare, NJ, USA), and then captured and analyzed by using the Las-3000 luminescent Image Analyzer (Fuji Film, Tokyo, Japan).
First, the effect of different concentrations of PT on the viability of CRC cells was evaluated by using the MTT assay. SW620 cells were treated with various concentrations (0, 5, 10, 20, and 40 µM) of PT for 24 hours. As shown in Fig. 1A, PT exerted a dose-dependent antiproliferative effect on SW620 cells. The FACScan analysis was used to determine the apoptotic cell fraction in SW620 cells treated with PT at concentrations of 5, 10, and 20 µM. It was confirmed that the sub-G1 fraction was elevated by PT treatment, suggesting that PT treatment promotes the apoptotic cell death of SW620 cells (Fig. 1B).
To verify that PT blocked cell motility, cell migration was evaluated using wound healing and Transwell assays. As shown in Fig. 2A, while cell migration was promoted after 24 hours of treatment, the migratory ability of cells was inhibited by PT treatment in a dose-dependent manner. The migration results using Transwell chamber showed that SW620 cells migrated to the lower side of the chamber after 24 hours; however, PT treatment significantly inhibited cell migration in a concentration-dependent manner (Fig. 2B, upper panel). The quantification of migrating cells by crystal violet staining demonstrated that 20 µM PT significantly inhibited the migratory ability of SW620 cells by approximately 80%.
Furthermore, the effect of PT on cell invasion was evaluated by using Matrigel as an extracellular matrix component. Invading cells were quantified by staining and it showed that 20 µM PT significantly inhibited the invasive ability of SW620 cells by approximately 80% (Fig. 2B, lower panel). These results demonstrate that PT inhibits cell migration and invasion.
To elucidate the underlying mechanism of the effect of PT on cell migration and invasion, the expression of related proteins was analyzed in SW620 cells by Western blotting (Fig. 3). Treatment with PT significantly upregulated the expression of E-cadherin (epithelial marker), while mesenchymal markers (vimentin, β-catenin, and Snail) were downregulated. Consistently, to determine whether PT regulates MMPs, which are powerful modulators of cell migration and invasion, we analyzed the protein level of MMP-2 and MMP-9 in cell extracts.19 PT inhibited the protein expression of MMP-2 and MMP-9 in a dose-dependent manner. The overexpression of COX-2 in CRC cells promotes invasive phenotype such as proliferation, motility invasion, and angiogenesis.20,21 Hence, the expression level of COX-2 in protein extracts was measured, and it was confirmed that PT treatment reduced the protein level of COX-2 in a concentration-dependent manner. These results suggest that PT treatment inhibits cell migration/invasion via the regulation of EMT markers, MMPs, and COX-2 expressions.
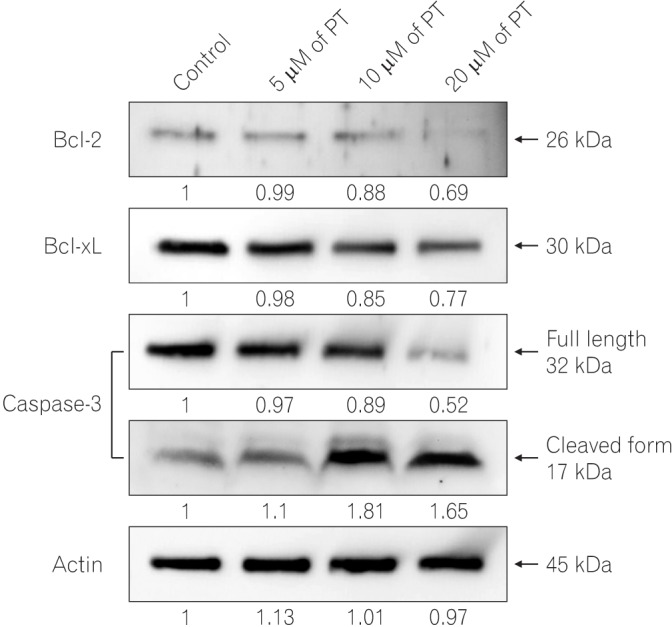
After establishing that PT induces apoptotic cell death in SW620 cells, we investigated whether the activation of several apoptosis-related proteins contributed to PT-induced apoptosis (Fig. 4). Among the antiapoptotic protein, the level of Bcl-2 and Bcl-xL proteins in SW620 cells decreased in a dose-dependent manner. While the level of pro-caspase-3 decreased after PT treatment, cleaved caspase-3 levels increased in a dose-dependent manner. Taken together, these results indicate that PT-induced apoptosis is caspase-dependent.
Metastasis is the major cause of death in cancer patients. Tumor cell migration and invasion from the surrounding tissue to the circulation via a process known as EMT is characterized as the early step of the metastatic process.22 Therefore, an agent that can effectively inhibit cancer cell proliferation, migration, and invasion has the potential to be developed as a new drug for preventing or treating progressive and metastatic cancers. To the best of our knowledge, this study was the first to demonstrate that PT exerts apoptotic effect and inhibits cells migration/invasion via the downregulation of associated markers including EMT markers. These results indicate that PT is a promising therapeutic agent for CRC patients with metastasis.
Recently, PT was reported to be involved in multiple pathways of apoptosis in various types of human cancer cells.12,23,24,25,26 In our previous study, PT induced apoptosis via the Bcl-2 family-dependent pathway, and it exerted antiangiogenesis effect via the downregulation of VEGF/VEGFR in human CRC in vitro and in vivo.13,27 However, further studies are still required to establish the underlying mechanism of the inhibitory effect of PT on CRC progression and metastasis. In addition, the effect of PT on CRC cell migration and invasion remains to be determined. In this study, we tried to find new factors involved in its mechanism and examined its functional role in CRC cell migration and invasion.
PT is the principal component of sesquiterpene lactones present in medicinal plants. PT contains an α-methylene-γ-lactone ring, which is a significant characteristic of sesquiterpene lactones. Sesquiterpene lactones are a large group of natural compound found in plants, with over 5,000 structures reported to date.28,29 A number of studies have reported the inhibitory effect of sesquiterpene lactones on cell migration/invasion and the EMT process in human cancer cells. Antrocin exhibited inhibitory activity on the growth, migration, and invasion of human bladder cancer cells.30 Codonolactone also attenuated transforming growth factor-β1-mediated EMT and motility of breast cancer cells.31 Moreover, it has been reported that costunolide, derived from Saussurea lappa, induces apoptosis and inhibits cell migration and invasion in neuroblastoma cells.32 To date, only two studies have demonstrated that PT suppresses cell migration and invasion in human cancer cells. Liu et al.17 showed that PT inhibits the migration of pancreatic cancer cells using the wound healing assay. In 2014, Kwak et al.18 reported that PT inhibits FAK-mediated cell invasion. However, the underlying mechanism or related signaling pathway of the suppressive effect of PT on cell migration/invasion and the EMT process has not been reported. In this study, we identified that PT inhibits CRC cell migration/invasion via the regulation of EMT markers. Our results were consistent to recent findings, and demonstrated the inhibitory effect of PT on cell motility and highlighted the functional role of PT in the EMT process.
COXs are key enzymes in the biosynthesis of PG during the inflammatory response and cancer progression. COX-2, the inducible form of COX, is overexpressed in early and advanced CRC, and it is associated with a poor prognosis.20 Overexpression of COX-2 promotes transformed and invasive phenotypes such as cell proliferation, cell migration/invasion, angiogenesis, resistance to apoptosis, and tumor growth. On the contrary, deletion of COX-2 in mice significantly inhibited the numbers of intestinal polyp and resulted in decreased neoplastic growth.21 Moreover, COX-2 has been shown to be linked to EMT in several cancer cells.33,34,35 Therefore, COX-2 plays a key role in cancer progression, development, and metastasis. Our study revealed that the mechanism by which PT suppresses CRC tumorigenesis is related to the regulation of COX-2 expression. This finding provides a deeper understanding of PT as a cancer therapy.
MMPs are a series of calcium-dependent zinc-containing endopeptidases.36 Among the MMP family, MMP-2 and MMP-9 play important roles in cancer progression by degrading the extracellular matrix, thereby allowing cancer cells to migrate out of the primary tumor to form metastasis. More specifically, MMP-2 and MMP-9 are capable of degrading type IV collagen, the most abundant component of the basement membrane, which is an essential step in the metastatic progression of most cancers.37 Western blotting results showed that MMP-2 and MMP-9 protein levels were down-regulated; thereby, further understanding the mechanism in which PT inhibits CRC cell migration and invasion.
Recent studies including our previous studies have suggested that multiple pathways are involved in PT-induced apoptotic cell death in human cancer cells, including oxidative stress, intracellular thiol depletion, endoplasmic reticulum stress, caspase activation, and mitochondrial dysfunction.11,12 Our present results were similar to a previous study in that PT induces apoptotic cell death by promoting mitochondrial dysfunction and inhibiting antiapoptotic proteins Bcl-2 and Bcl-xL. Moreover, PT treatment stimulates the activation of caspase-3, which leads to an irreversible apoptotic stage. These results have suggested that PT induced apoptosis via the caspase-dependent mitochondrial pathway.
Taken together, we demonstrated that PT induces apoptotic cell death via the downregulation of Bcl-2 family members and activation of caspase-3. Our experiments also provide the evidence that PT inhibits cell migration/invasion via the regulation of EMT markers, MMPs, and COX-2 involved in cancer progression and tumorigenesis. These findings provide a better understanding of the pharmacological effects of PT and supports PT as a promising approach for the treatment of CRC.
NOTES
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.PMID: 21296855.


2. Bosetti C, Levi F, Rosato V, et al. Recent trends in colorectal cancer mortality in Europe. Int J Cancer 2011;129:180-191.PMID: 20824701.


3. Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544-573.PMID: 19998273.



4. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-117.PMID: 24639052.


5. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29.PMID: 22237781.


6. Bork PM, Schmitz ML, Kuhnt M, Escher C, Heinrich M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kappaB. FEBS Lett 1997;402:85-90.PMID: 9013864.


7. Murphy JJ, Heptinstall S, Mitchell JR. Randomised double-blind placebo-controlled trial of feverfew in migraine prevention. Lancet 1988;2:189-192.PMID: 2899663.


8. Hehner SP, Heinrich M, Bork PM, et al. Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J Biol Chem 1998;273:1288-1297.PMID: 9430659.


9. Oka D, Nishimura K, Shiba M, et al. Sesquiterpene lactone parthenolide suppresses tumor growth in a xenograft model of renal cell carcinoma by inhibiting the activation of NF-kappaB. Int J Cancer 2007;120:2576-2581.PMID: 17290398.


10. Kishida Y, Yoshikawa H, Myoui A. Parthenolide, a natural inhibitor of nuclear factor-kappaB, inhibits lung colonization of murine osteosarcoma cells. Clin Cancer Res 2007;13:59-67.PMID: 17200339.


11. Zhang S, Ong CN, Shen HM. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett 2004;208:143-153.PMID: 15142672.


12. Wen J, You KR, Lee SY, Song CH, Kim DG. Oxidative stress-mediated apoptosis: the anticancer effect of the sesquiterpene lactone parthenolide. J Biol Chem 2002;277:38954-38964.PMID: 12151389.


13. Kim SL, Trang KT, Kim SH, et al. Parthenolide suppresses tumor growth in a xenograft model of colorectal cancer cells by inducing mitochondrial dysfunction and apoptosis. Int J Oncol 2012;41:1547-1553.PMID: 22895542.


14. Kim SL, Liu YC, Park YR, et al. Parthenolide enhances sensitivity of colorectal cancer cells to TRAIL by inducing death receptor 5 and promotes TRAIL-induced apoptosis. Int J Oncol 2015;46:1121-1130.PMID: 25502339.


15. Kanthan R, Senger JL, Kanthan SC. Molecular events in primary and metastatic colorectal carcinoma: a review. Patholog Res Int 2012;2012:597497PMID: 22997602.




16. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15:178-196.PMID: 24556840.




17. Liu JW, Cai MX, Xin Y, et al. Parthenolide induces proliferation inhibition and apoptosis of pancreatic cancer cells in vitro. J Exp Clin Cancer Res 2010;29:108PMID: 20698986.



18. Kwak SW, Park ES, Lee CS. Parthenolide induces apoptosis by activating the mitochondrial and death receptor pathways and inhibits FAK-mediated cell invasion. Mol Cell Biochem 2014;385:133-144.PMID: 24065392.



19. Toth M, Sohail A, Fridman R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol Biol 2012;878:121-135.PMID: 22674130.


20. Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol 2005;23:2840-2855.PMID: 15837998.


22. Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 2006;172:973-981.PMID: 16567498.



23. Carlisi D, D'Anneo A, Angileri L, et al. Parthenolide sensitizes hepatocellular carcinoma cells to TRAIL by inducing the expression of death receptors through inhibition of STAT3 activation. J Cell Physiol 2011;226:1632-1641.PMID: 21413021.


24. Dai Y, Guzman ML, Chen S, et al. The NF (nuclear factor)-kappaB inhibitor parthenolide interacts with histone deacetylase inhibitors to induce MKK7/JNK1-dependent apoptosis in human acute myeloid leukaemia cells. Br J Haematol 2010;151:70-83.PMID: 20701602.



25. Nakshatri H, Rice SE, Bhat-Nakshatri P. Antitumor agent parthenolide reverses resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene 2004;23:7330-7344.PMID: 15286701.



26. Wu C, Chen F, Rushing JW, et al. Antiproliferative activities of parthenolide and golden feverfew extract against three human cancer cell lines. J Med Food 2006;9:55-61.PMID: 16579729.


27. Kim SL, Lee ST, Trang KT, et al. Parthenolide exerts inhibitory effects on angiogenesis through the downregulation of VEGF/VEGFRs in colorectal cancer. Int J Mol Med 2014;33:1261-1267.PMID: 24573421.


28. Amorim MH, Gil da Costa RM, Lopes C, Bastos MM. Sesquiterpene lactones: adverse health effects and toxicity mechanisms. Crit Rev Toxicol 2013;43:559-579.PMID: 23875764.


29. Chadwick M, Trewin H, Gawthrop F, Wagstaff C. Sesquiterpenoids lactones: benefits to plants and people. Int J Mol Sci 2013;14:12780-12805.PMID: 23783276.



30. Chiu KY, Wu CC, Chia CH, Hsu SL, Tzeng YM. Inhibition of growth, migration and invasion of human bladder cancer cells by antrocin, a sesquiterpene lactone isolated from Antrodia cinnamomea, and its molecular mechanisms. Cancer Lett 2016;373:174-184.PMID: 26679052.


31. Fu J, Ke X, Tan S, et al. The natural compound codonolactone attenuates TGF-beta1-mediated epithelial-to-mesenchymal transition and motility of breast cancer cells. Oncol Rep 2016;35:117-126.PMID: 26549400.


32. Tabata K, Nishimura Y, Takeda T, Kurita M, Uchiyama T, Suzuki T. Sesquiterpene lactones derived from Saussurea lappa induce apoptosis and inhibit invasion and migration in neuroblastoma cells. J Pharmacol Sci 2015;127:397-403.PMID: 25953266.


33. Qin G, Xu F, Qin T, et al. Palbociclib inhibits epithelial-mesenchymal transition and metastasis in breast cancer via c-Jun/COX-2 signaling pathway. Oncotarget 2015;6:41794-41808.PMID: 26540629.



34. Li ZL, Ye SB, OuYang LY, et al. COX-2 promotes metastasis in nasopharyngeal carcinoma by mediating interactions between cancer cells and myeloid-derived suppressor cells. Oncoimmunology 2015;4:e1044712PMID: 26451317.



35. Wang KH, Kao AP, Chang CC, Lin TC, Kuo TC. Bisphenol Ainduced epithelial to mesenchymal transition is mediated by cyclooxygenase-2 up-regulation in human endometrial carcinoma cells. Reprod Toxicol 2015;58:229-233.PMID: 26546977.


36. Verma RP, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem 2007;15:2223-2268.PMID: 17275314.


37. Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta 2004;1705:69-89.PMID: 15588763.


Fig. 1
Parthenolide (PT) decreases the viability of colorectal cancer cells and induces apoptotic cell death. (A) SW620 cells were treated with PT at concentrations of 0, 5, 10, 20, or 40 µM. After 24 hours of incubation, the MTT assay was conducted to measure viability. Data represent the mean±SE of three independent experiments. (B) Changes in the cell cycle induced by PT treatment. After treatment with PT for 24 hours, SW620 cells were harvested and stained with propidium iodide. The percentage of sub-G1 population is shown in each histogram, and the total number of events analyzed for each condition was 10,000. aP<0.05, bP< 0.01 versus control.
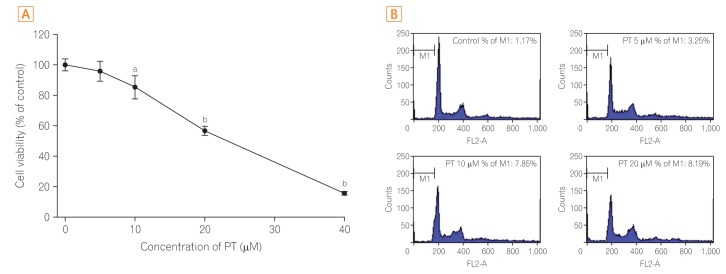
Fig. 2
Parthenolide (PT) inhibits colorectal cancer cell migration and invasion in vitro. (A) Cell mobility as detected by the wound healing assay. Cells were grown to confluence in 6-well plates, wounded, and treated with the indicated concentrations of PT for 24 hours. Scratch closure was monitored for 24 hours; microscopic images taken at 0 and 24 hours postscratching are shown. Images were captured at a magnification of ×10, and the columns represent the means±SEs of three independent experiments. (B) Migration and invasion assays were performed by the Transwell cell culture system. After incubating for the indicated time, the cells were fixed and stained with crystal violet. Microscopic images were captured at ×20 magnification, and the columns represent the means±SEs of three independent experiments. aP<0.01, bP<0.05 versus the width at 0 hour; cP<0.05, dP<0.01 versus control.

Fig. 3
Parthenolide (PT) inhibits epithelial-mesenchymal transition-associated markers. Cells were treated with different concentrations of PT, and total cell lysates were extracted by using RIPA buffer. The levels of E-cadherin, β-catenin, vimentin, Snail, matrix metalloproteinase-2 (MMP-2), MMP-9, cyclooxygenase-2 (COX-2), and actin were assessed by Western blotting with the appropriate antibodies. Actin was used as a loading control.
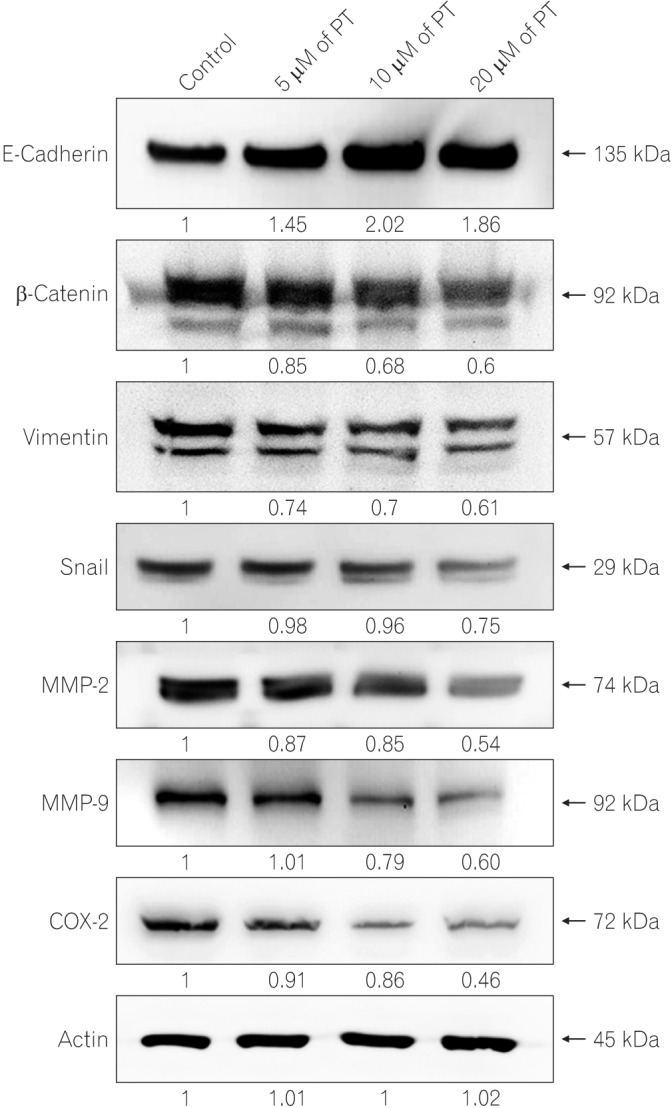
Fig. 4
Parthenolide (PT) regulates Bcl-2 family members and caspase-3 activation. The cells were treated with different concentrations of PT, and total cell lysates were extracted by using RIPA buffer. The levels of Bcl-2, Bcl-xL, caspase-3, and actin were assessed by Western blotting with the appropriate antibodies. Actin was used as a loading control.
- TOOLS






