 |
 |
- Search
| Intest Res > Volume 16(2); 2018 > Article |
|
Abstract
Background/Aims
Decreased trough levels of infliximab (TLI) and antibodies to infliximab (ATI) are associated with loss of response (LOR) in Crohn's disease. Two prospective studies were conducted to determine whether TLI or ATI better correlates with LOR (Study 1), and whether TLI could become a predictor of mucosal healing (MH) (Study 2).
Methods
Study 1 was conducted in 108 patients, including those with LOR and remission to compare ATI and TLI in discriminating the 2 conditions based on receiver operating characteristic (ROC) curve analyses. Study 2 involved 35 patients who were evaluated endoscopically.
Results
In Study 1, there were no differences between the 2 assays in ROC curve analyses; the TLI cutoff value for LOR was 2.6 ┬Ąg/mL (sensitivity, 70.9%; specificity, 79.2%), and the ATI cutoff value was 4.9 ┬Ąg/mL (sensitivity, 65.5%; specificity, 67.9%). The AUROC (area under the ROC curve) of TLI was greater than that of ATI. AUROC was useful for discriminating between the 2 conditions. In Study 2, the TLI was significantly higher in the colonic MH group than in the non-MH group (2.7 ┬Ąg/mL vs. 0.5 ┬Ąg/mL, P=0.032).
Infliximab (IFX) is a chimeric antibody preparation against tumor necrosis factor ╬▒, and, although it demonstrates a strong therapeutic effect in CD, loss of response (LOR) occurs in about 30% to 50% of patients during IFX maintenance therapy after remission induction.1,2 The presence of antibodies to IFX (ATI), which correlate strongly to infusion reactions, is believed to be a factor inducing LOR.3 However, there are few detailed comparisons of whether trough level of IFX (TLI) or ATI is useful in determining LOR.4,5 Moreover, the goal of CD treatment has recently been shifting away from achieving clinical remission through IFX treatment and toward mucosal healing (MH), though the TLI required to achieve this goal has yet to be established.
Accordingly, in the present study, we conducted a prospective trial to determine whether TLI or ATI is more effective in judging LOR. We also conducted a prospective trial of whether TLI is associated with achieving MH.
The present study was a single-site, prospective study that was conducted in 215 CD patients who received IFX maintenance therapy (IFX infusions [5 or 10 mg/kg] every 6 to 8 weeks) at Fukuoka University Chikushi Hospital, Department of Gastroenterology, between November 2012 and November 2014. The protocol was approved by the Institutional Review Board for Clinical Research of Fukuoka University Chikushi Hospital (November 2012, R12-036).
Subjects were patients 18 years and older in whom initial treatment induced remission, were undergoing maintenance therapy, and had been receiving IFX treatment for more than 14 weeks and no longer than 5 years. The IFX dose (IFX, 5 or 10 mg/kg) and concomitant immunomodulatory use were not criteria for exclusion.
In addition, the TLI and ATI measurements used in this study and the assessment of endoscopic mucosal activity were performed blind, without knowledge of the results of either.
A total of 108 patients were enrolled in Study 1, in which the objective was to investigate the relationships of TLI and ATI with the clinical demographics. In Study 2, 35 patients were enrolled to investigate the relationships of TLI with endoscopic MH. The inclusion criteria for each of these studies are shown in Fig. 1. Study 1 included 108 patients and Study 2 included 35 patients who met the following criteria: (1) efficacy of initial infusion of IFX was response; (2) provided informed consent to blood sampling to measure IFX blood concentrations and to endoscopy; (3) their course could be followed up sufficiently; (4) their CDAI could be measured; and (5) were able to undergo colonoscopy (CS) or double-balloon enteroscopy (DBE) within 2 months before or after the date of IFX blood concentration measurement. Exclusion criteria were: (1) continuous administration of IFX for Ōēż14 weeks or >5 years (49 patients); (2) a stoma (19 patients); or (3) not obtaining consent (4 patients).
In the design of Study 1, the first assay (assays A and B) was performed with patients divided clinically using CDAI into a remission group and a LOR group after patient enrollment. Then, after about 1 year, we conducted a follow-up evaluation of ATI-positive patients in the remission group whose course had been closely followed. In Study 2, endoscopic examination and the first assay (assay A) were performed within 2 months of each other.
Eleven of the 108 patients (10.2%) in Study 1 were receiving an IFX dose of 10 mg/kg. Nine of the 35 patients (25.7%) in Study 2 were receiving an IFX dose of 10 mg/kg.
Serum taken immediately before IFX infusion was used for TLI measurements. TLI measurements were conducted at Tanabe R&D Service Co., Ltd. (Saitama, Japan; assay A), and Shiga University of Medical Science (assay B). Measurements were performed blind, without disclosing patient background or clinical results.
Serum TLI measurements with assay A were conducted with an ELISA using a monoclonal antibody against IFX obtained from Jansen Biotech Inc. (Horsham, PA, USA). The detection limit was 0.1 ┬Ąg/mL.6
Serum TLI measurements with assay B were conducted using an ELISA system using an avidin ELISA plate (blocking-less type; Sumitomo Bakelite Co., Ltd., Tokyo, Japan).7
With assay A, measurements were performed using an ELISA method based on a double-antigen format. If IFX is present in the blood it will compete with the labeled-IFX, making accurate measurement of ATI impossible. As a result, to obtain a positive or negative result for ATI, the determination can only be made under conditions in which IFX is not present in the blood.
On the other hand, with assay B, ATI measurements were conducted using an original method developed by Shiga University of Medical Science called modified Direct-ELISA.8 In modified direct-ELISA, the IFX-ATI immune complexes were initially dissociated, and the binding capacities of ATIs were recovered. ATIs were then immobilized onto ELISA plates and detected with horseradish peroxidase-labeled IFX.
Biochemical markers such as CRP were measured by the Laboratory Test Department of Fukuoka University Chikushi Hospital. Blood samples taken immediately before IFX infusion were also used for these measurements.
The clinical activity index for IFX was assessed according to the CDAI.9 A CDAI Ōēż150 indicates a clinically inactive state, while Ōēź150 indicates the active phase. In this study, the CDAI was measured at the time IFX trough levels were measured, following infusion of IFX.
In Study 1, because the objective was to evaluate the clinical usefulness for diagnosing LOR, patients were classified as LOR or remission strictly based on the CRP level and CDAI score at the time IFX blood concentrations were measured. Remission was defined as CDAI <150 points and CRP <0.3 mg/dL. LOR was defined as CDAI Ōēź150 points and/or CRP Ōēź0.3 mg/dL.
The DBE models used were the Fujinon EN-580T, EN-450P5, and EN-450T5 (Fujinon Inc., Saitama, Japan); the CS models used were the Olympus PCF-240AI, PCF-PQ260I, PCF-Q260AI, and PCF-290I (Olympus, Tokyo, Japan). A transanal approach was used in all patients. DBE was performed in 18 patients, and CS was performed in 17 patients. The mean distance of small intestinal observation after passing through Bauhin's valve was 62 cm (range, 7-150 cm). Lesions were assessed at the site where activity was the strongest that could be confirmed endoscopically.
The Fukuoka index was used to evaluate endoscopic mucosal activity. There are essentially 3 components to this index: stenosis, polyposis, and ulcer.10 In this study, ulcer scores were used to assess ileal and colorectal mucosa. Without using the polyposis score, Beppu et al.11 reported no link between the stenosis score and MH assessment. For ileal and colorectal lesions, the sites where activity was the highest were assessed for the activity index. No lesion (0 point) or ulcer scarring (1 point) was defined as ŌĆ£mucosal healing (MH),ŌĆØ and an ulcer score of 2 to 4 points was defined as ŌĆ£non-mucosal healing (nMH).ŌĆØ For small intestinal lesions, the sites evaluated were the small intestinal mucosa in patients with ileitis CD and ileocolitis CD. For colonic lesions, the colonic mucosa in colitis CD and the colonic mucosa in ileocolitis CD were the sites evaluated.
Fisher exact test or the Mann-Whitney U-test was used in 2-group comparisons, and to analyze the diagnostic ability of TLI and ATI, cutoff values were established for each using the minimum distance criteria from the area under the receiver operating characteristic curve (AUROC). Significance was defined as a P-value Ōēż0.05. Statistical analysis was performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA).
Based on CDAI and CRP, the 108 CD patients were categorized and placed in either the LOR group or the remission group. All patients who were enrolled in this study received IFX for at least 1 year regardless of LOR because no patient experienced clinically significant exacerbation of CD during IFX maintenance within 1 year. The characteristics of the patients in the 2 groups are shown in Table 1. There were no clear significant differences in the male to female ratio, age at initial IFX infusion, surgical history, or anal lesions. However, disease duration was slightly longer in the LOR group than in the remission group (9.5 years vs. 6.8 years, P=0.0512). Ileocolitis tended to be the most common type of disease in both groups. The duration of IFX treatment was approximately 3 years in both groups (3.1 years vs. 3.6 years, P=0.0658). There were no significant differences in the concomitant medications used at the time IFX blood concentrations were measured. IFX 10 mg/kg infusions were used significantly more frequently in the LOR group than in the remission group (18.2% vs. 1.9%, P=0.0082).
TLI was compared in the LOR and remission groups using both assay A and assay B. The overall results showed no differences between the groups in TLI (LOR: 2.4┬▒3.2 ┬Ąg/mL vs. 2.3┬▒2.7 ┬Ąg/mL, P=0.33; remission: 5.3┬▒4.2 ┬Ąg/mL vs. 5.2┬▒3.8 ┬Ąg/mL, P=0.85) (Supplementary Fig. 1). When analyzed by assay, values were significantly lower in the LOR group than in the remission group with each assay (assay A: 2.4┬▒3.2 ┬Ąg/mL vs. 5.3┬▒4.2 ┬Ąg/mL, P<0.0001; assay B: 2.3┬▒2.7 ┬Ąg/mL vs. 5.2┬▒3.8 ┬Ąg/mL, P<0.0001) (Supplementary Fig. 2).
The results of ATI measured in assay A are shown in the LOR and remission groups. The numbers of ATI positive and ATI negative patients were small in both the LOR group and the remission group. Many patients in both the LOR group and the remission group were inconclusive ATI. Moreover, no statistical relationship was seen between the presence of ATI and LOR (P<0.1) (Supplementary Fig. 3).
Next, 108 patients whose ATI values were measured using assay B are shown in the LOR and remission groups. A comparison of ATI levels in the LOR and remission groups showed that ATI was significantly higher in the LOR group (18.4┬▒30.1 ┬Ąg/mL vs. 6.5┬▒9.2 ┬Ąg/mL, P=0.0014) (Supplementary Fig. 4).
TLI and ATI values as determined assay B were compared using AUROC to determine their associations with LOR (Fig. 2). The LOR cutoff value for TLI was 2.6 ┬Ąg/mL (sensitivity, 70.9%; specificity, 79.2%; PPV, 77.6%; NPV, 71.2%), while that for ATI was 4.9 ┬Ąg/mL (sensitivity, 65.5%; specificity, 67.9%; PPV, 67.9%; NPV, 65.5%).
First, using the ATI results from the previous assay A and the ATI cutoff value results from assay B, we compared the sensitivity, specificity, positive predictive value, and negative predictive value for ATI from assays A and B. The results showed that the sensitivity, specificity, and negative predictive value were lower in assay A than in assay B (Supplementary Tables 1 and 2).
Next, a comparison of the AUROC for TLI and ATI revealed that the AUROC of TLI was larger than that of ATI (77.8% vs. 67.9%). These results showed that TLI has a high capacity for discrimination.
The percentage of patients positive for ATI in the LOR and remission groups was also investigated (Fig. 3). With assay A, it was not possible to accurately compare ATI-positive and ATI-negative cases, because a relatively large number of patients were inconclusive for ATI. Looking at the results of assay B, the rate of ATI was seen to be significantly higher in the LOR group than in the remission group (65.5% vs. 32.1%, P=0.0006). However, these data also showed that ATI was positive in a high percentage (32.1%) of the remission group (ŌĆ£ATI positiveŌĆØ was defined as ATI positivity on assay A and ATI of Ōēź4.9 ┬Ąg/mL on assay B).
In the remission group, patients with an ATI Ōēź4.9 ┬Ąg/mL with assay B (n=17) were categorized as ATI-positive, and those with ATI Ōēż4.9 ┬Ąg/mL (n=36) were categorized as ATI-negative, and the incidence of infusion reactions (IRs), incidence of LOR, and percent decrease in TLI were investigated in 51 patients whose clinical course could be followed in detail after 1 year (17 cases with positive ATI and 34 cases with negative ATI). Serological follow-up was possible in 20 patients (17 cases with positive ATI and 3 cases with negative ATI). An IR was defined as unable to continue IFX. LOR was defined as in Study 1 and was evaluated by measuring CDAI and CRP after 1 year. A TLI decrease was defined as a Ōēź50% decrease in TLI from the initial measurements. The results of these investigations showed that IR occurred in 3 of 17 ATI-positive patients (17.6%) and 1 of 34 ATI-negative patients (2.9%) after 1 year of follow-up (P=0.0967). IR tended to occur more readily in ATI-positive patients, but otherwise no differences were observed in the incidence of LOR had occurred in 1 of 15 ATI-positive patients (6.7%) and 1 of 34 ATI-negative patients (2.9%) (P=0.523), or TLI had decreased Ōēź50% in 1 of 13 ATI-positive patients (7.7%) and in 0 of 7 ATI-negative patients (0%) (P=1.0000).
In addition, 3 of the 17 patients who were positive for ATI and 1 of the 34 patients negative for ATI discontinued IFX or switched to ADA due to an IR. However, during the course of 1 year, ATI-positive patients did not trend in a major way toward LOR (Supplementary Fig. 5).
Based on the results of Study 1, because TLI showed a better ability to discriminate than ATI, and there were no differences between the assay methods in terms of the results, the relationship between TLI and mucosal assessment was investigated using assay A.
Table 2 shows the characteristics of the 35 CD patients of Study 2. The cohort trended toward patients with a relatively young age at diagnosis (22 years), by sex toward men, and by disease type toward ileocolitis. IFX treatment duration was approximately 3 years. Time between measurement of TLI and the endoscopy procedure was 0.3 months. In contrast to CDAI, which was in a state of remission at the time of IFX measurement, CRP levels were high (CDAI, 122 and CRP, 0.9 mg/dL). With regard to concomitant therapy, 8 patients (22.9%) were receiving Ōēź900 kcal/day enteral nutrition, and 15 patients (42.9%) were taking an immunomodulator.
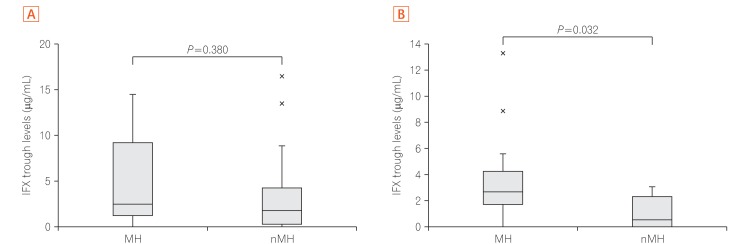
The 31 patients who had small intestinal lesions were classified and assigned to the MH group (10 patients) or the nMH group (21 patients) (Fig. 4a). Comparison of patients with small intestinal lesions revealed no significant difference between the MH and nMH groups in terms of TLI (2.5 ┬Ąg/mL vs. 1.8 ┬Ąg/mL, P=0.38). No relationship between the MH group and nMH group was seen with regard to patients taking IFX 10 mg/kg (30.0% vs. 23.3%, P=1.000) or patients positive for ATI (10.0% vs. 14.3%, P=1.000), nor was statistical significance seen between the presence of ATI and MH (P=0.5463).
Next, the 21 patients with large intestinal lesions were classified and assigned to either the MH group (13 patients) or the nMH group (8 patients) (Fig. 4b). TLI levels were significantly higher in the MH group than in the nMH group (2.7 ┬Ąg/mL vs. 0.5 ┬Ąg/mL, P=0.032). However, no relationship between the MH group and the nMH group was seen with regard to patients taking IFX 10 mg/kg (15.4% vs. 37.5%, P=0.3254) or patients positive for ATIs (0% vs. 25.0%, P=0.3333), and no statistically significant difference was seen between the presence of ATI and MH (P=0.1328).
Based on the above, it was concluded that colorectal MH is obtained with TLI Ōēź2.7 ┬Ąg/mL.
We investigated whether there were any significant differences between the MH group and the nMH group in small intestine and colon lesions. There were no background factors that showed a significant difference between the MH group and the nMH group for small intestinal lesions. For the colon, however, a tendency was seen for the CRP value to be lower in the MH group than in the nMH group (0.5┬▒0.6 ┬Ąg/mL vs. 0.9┬▒1.0 mg/dL, P=0.0597) (Supplementary Table 3).
This prospective study examined whether TLI or ATI was useful for the evaluation of LOR. It was found that TLI was statistically more useful than ATI in judging LOR. Furthermore, in the relationship between MH and TLI, TLI was shown to be useful in MH of the large intestine. This is the first report to separately assess MH in the large and small intestines.
In the clinical background for this study, the mean period from the start of IFX until assay or endoscopy was performed was about 3 years. In a report by Beppu et al.,11 the IFX treatment course was relatively long, at about 4 years. In other recent reports IFX was also administered for long durations of 40.0 to 68.7 months.12,13 The treatment period is therefore consistent between these reports and that in Studies 1 and 2 described herein.
In Study 1, TLI or ATI was useful for discrimination of LOR was determined using AUROC analysis.
There are various methods for the measurement of TLI and ATI. However, there have been few reports that use 2 or more measuring methods in a clinical trial. The present study compared the results of 2 typical measurement methods (Tanabe R & D, assay A; Shiga University of Medical Science, assay B) using the same serum. This was done because there is a significant problem in ATI measurement with assay A. It is known that there are inconclusive cases in which ATI cannot be measured, although it can be measured in 54% to 70% of cases; there are cases, however, that do not satisfy the requirements of assay A for measuring ATI.3,6,14 Therefore, development of a method to measure ATI that does not depend on the serum IFX density was urgently needed. Imaeda et al.8 may have solved this problem by raising the sensitivity of the ATI value using a new measurement method (Direct ELISA; DA ELISA and IC-based ELISA). The present study examined the clinical value of ATI measurement using this method and the DA ELISA method.
The results showed that assay A had clearly lower sensitivity, specificity, and negative predictive value for ATI than assay B. In the LOR group, the ATI positive rate was higher in assay B than in assay A. We therefore judged that clinical activity could not be satisfactorily evaluated with the ATI results from assay A.
In addition, the ATI level in assay B was significantly higher in the LOR group, while TLI levels were significantly lower in the LOR group than in the remission group. These results show that the presence of ATI is related to a drop in TLI when the ATI level does not depend on serum IFX, as is the case with assay B. However, there have been few reports that compared TLI and ATI evaluations of clinical LOR with high discrimination ability. AUROC analysis is useful for evaluating discrimination ability. There have been only 3 reports, including the present study that compared TLI with ATI by AUROC analysis (Table 3).4,5 In both of the other reports, the AUROC of TLI was larger than the AUROC of ATI. Those results are not inconsistent with this study. This is certain to depend on the measurement technique of ATI and has the possibility of greatly contributing to the clarification of LOR. However, the present results suggest that measurement of TLI alone is sufficient for the evaluation of clinical LOR. ATI appears to have a supplementary role; it may be appropriate to measure ATI when the TLI value is low and clinical LOR is suspected or when an IR may have occurred. A low ATI did not affect the long-term prognosis when the investigation of ATI positivity included many cases of ATI positivity in the remission group in the present study and in the remission group at 1year.
In Study 2, the relationship between endoscopic MH and TLI was examined based on the results of Study 1. The TLI necessary for small intestinal MH and colonic MH was examined in this study based on the supposition that it was different. In a recent report MH was defined using a different endoscopic score than that used in the present study. However, most reports define MH as disappearance of the ulcer lesion. The definition of MH in the present study used the ulcer score of the Fukuoka index. The reason why this definition was used in the present study was that, using the Fukuoka index, Beppu et al.11 enumerated the points for calculating the scores at which the small intestinal lesions and the colon change to a morbid state were separately appreciable. In addition, it was reported that small intestinal MH and colonic MH were related to clinical remission. The results of Study 2 appeared to show that colonic MH and TLI were causally related, while there was no significant relationship between small intestinal MH and TLI.
The reasons why no significant differences were seen in TLI and MH in small intestinal lesions are thought to be the following: (1) endoscopic observation is easy in large intestinal lesions and detailed lesions can be identified. As a result, findings that agree with clinical symptoms can be obtained. With small intestinal lesions, however, it is not easy to observe the entire small intestine and the lesion areas are small. It is possible that clinical symptoms and small intestinal lesions do not agree because of the tendency to identify very small lesions. (2) The effectiveness of IFX for small intestinal lesions may be lower than that in the large intestine. Imaeda et al.12 reported that TLI of Ōēź4.0 ┬Ąg/mL was needed in MH. Additionally, Ungar et al.15 reported that 80% to 90% of patients achieve MH with a TLI of 6 to 10 ┬Ąg/mL and that the MH achievement rate becomes higher as the TLI value increases. Although those authors did not classify and score small intestinal lesions and large intestinal lesions, considering those reports our findings suggest that higher TLI is needed in order to achieve small intestinal MH. The above reasons may therefore explain why no significant differences were seen between small intestinal MH and TLI. At the same time, no significant relationship was seen between the MH group and the nMH group in either the small intestine or the large intestine with ATI, although this was a comparison using assay A. In the large intestine there was also a tendency to achieve MH when the CRP value was low, but no relationship was seen between other background factors and achieving MH. Imaeda et al.12 reported that ATI and MH had only a weak relationship; in the present study, MH and ATI also had a weak relationship.
Whether combined therapy with an immunomodulator is related to TLI was evaluated. TLI was not higher in LOR and remission groups even with combined use of an immunomodulator.
This study has several limitations. Although Study 1 was a prospective study, there was no follow up from the first IFX administration. Furthermore, with limitation to the cross-sectional period only patients receiving long-term IFX were enrolled. Recent reports have shown that ATI can exist as stable ATI or transient ATI, and that transient ATI sometimes appears coincidentally during the time a patient is receiving IFX and does not affect LOR. Stable ATI, however, is reported to affect LOR. Therefore, multiple ATI measurements are recommended as it cannot be determined whether ATI is stable or transient with a single measurement. There are also reports that in determining LOR, a more accurate prediction is possible with a combination of CRP, TLI, and stable ATI.16,17,18 As ATI was measured only once in this study, it could not be determined whether it was stable or transient. Moreover, ATI-positive patients in the remission group were taken to be patients who would not experience LOR in at least one year and in whom TLI would not significantly decrease; however, the possibility cannot be ruled out that many cases of transient ATI were also included. Nevertheless, from reports that transient ATI does not affect LOR and that multiple ATI measurements are recommended, we recommend measurement of TLI for clinical purposes. TLI measurement means that LOR can be determined with a single measurement, rather than having to perform multiple measurements to determine whether ATI is stable or transient.
The limitations of Study 2 are thought to be that the number of patients who could participate in the study was small and that the entire small intestine could not be observed.
In conclusion, the present study showed that TLI was more useful for diagnosis and evaluation of LOR in CD during IFX maintenance therapy than ATI; ATI appears to have a supporting role in LOR evaluation. In addition, remission could be evaluated only by TLI. As for colonic MH, a relationship with TLI was observed; for remission, TLI needed to be greater than 2.6 ┬Ąg/mL.
NOTES
FINANCIAL SUPPORT: This article was prepared with financial assistance from the Study Group on Intractable Disease, and Health and Labour Science Research Grants to chief researcher Dr. Suzuki from the Ministry of Health, Labour and Welfare of Japan.
AUTHOR CONTRIBUTION: A.K. and T.M. were involved in conception and design of the study. A.K. was also involved in acquisition and analysis of data. All authors were involved in interpretation of data, drafting and critical revision of the manuscript and have approved the final manuscript for submission. All authors also had full access to all the data in the study and had final responsibility for the decision to submit for publication.
SUPPLEMENTARY MATERIALS
Supplementary Fig. 1
Comparison of trough levels of infliximab (IFX) between assay A and assay B. Trough levels of IFX (TLI) were measured in the loss of response (LOR) (A) and remission (B) groups using assays A and B. Serum drawn immediately before IFX infusion was used for TLI measurements. Mean TLI values with assays A and B are 2.4┬▒3.2 ┬Ąg/mL vs. 2.3┬▒2.7 ┬Ąg/mL (P=0.33) in the LOR group (A), and 5.2┬▒4.2 ┬Ąg/mL vs. 5.2┬▒3.8 ┬Ąg/mL (P=0.85) in the remission group (B), respectively.
Supplementary Fig. 2
Comparison of trough levels of infliximab (IFX) between the loss of response (LOR) and remission groups. (A) Assay A and (B) assay B were used to measure trough levels of IFX (TLI) in both the LOR and remission groups. Serum drawn immediately before IFX infusion was used for TLI measurements. Mean TLI values in the LOR and remission groups are 2.4┬▒3.2 ┬Ąg/mL vs. 5.3┬▒4.2 ┬Ąg/mL (P<0.0001) with assay A and 2.3┬▒2.7 ┬Ąg/mL vs. 5.2┬▒3.8 ┬Ąg/mL (P<0.0001) with assay B, respectively.
Supplementary Fig. 3
Comparison of number of patients with loss of response (LOR) between antibodies to infliximab (ATI) positive, ATI negative and ATI inconclusive groups (Study 1). This is a graph of ATI assessed using assay A. No statistically significant in frequency of LOR was seen in 3 groups various ATI conditions (P=0.0676).
Supplementary Fig. 4
Comparison of antibodies to infliximab (ATI) levels by assay B in the loss of response (LOR) and remission groups. Assay B was used to measure ATI levels in both the LOR and remission groups (P=0.0014).
Supplementary Fig. 5
Follow-up of patients in remission group for 1 year after initial trough levels of infliximab (TLI) measurements. Patients in the remission group were separated into antibodies to infliximab (ATI)-positive (ATI >4.9 ┬Ąg/mL) and ATI-negative (ATI Ōēż4.9 ┬Ąg/mL) groups. The rates of infusion reaction were 17.6% in the ATI-positive group and 2.9% in the ATI-negative group (P=0.0967), and the loss of response (LOR) rates after 1 year were 6.7% and 2.9%, respectively (P=0.5230), while the proportions in whom TLI had decreased by Ōēź50.0% were 7.7% and 0%, respectively (P=1.0000).
Supplementary Table 1
Sensitivity, Specificity, PPV, NPV of ATI by assay A and assay B (Study 1)
Supplementary Table 2
ATI Positivity in LOR Group and Remission Group by Assay A and Assay B (Study 1)
Supplementary Table 3
Comparison of Characteristics of MH and nMH Groups in Small Intestine and Colon in Study 2
References
1. Gisbert JP, Pan├®s J. Loss of response and requirement of infliximab dose intensification in Crohn's disease: a review. Am J Gastroenterol 2009;104:760-767.PMID: 19174781.



2. Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn's disease. Aliment Pharmacol Ther 2011;33:987-995.PMID: 21366636.


3. Danese S, Fiorino G, Reinisch W. Review article: causative factors and the clinical management of patients with CrohnŌĆÖs disease who lose response to anti-TNF-alpha therapy. Aliment Pharmacol Ther 2011;34:1-10.PMID: 21539588.


4. Steenholdt C, Bendtzen K, Brynskov J, Thomsen O├ś, Ainsworth MA. Cut-off levels and diagnostic accuracy of infliximab trough levels and anti-infliximab antibodies in Crohn's disease. Scand J Gastroenterol 2011;46:310-318.PMID: 21087119.


5. Vande Casteele N, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in CrohnŌĆÖs disease. Gut 2015;64:1539-1545.PMID: 25336114.


6. Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998;41:1552-1563.PMID: 9751087.


7. Imaeda H, Takahashi K, Fujimoto T, et al. Clinical utility of newly developed immunoassays for serum concentrations of adalimumab and anti-adalimumab antibodies in patients with CrohnŌĆÖs disease. J Gastroenterol 2014;49:100-109.PMID: 23575576.


8. Imaeda H, Andoh A, Fujiyama Y. Development of a new immunoassay for the accurate determination of anti-infliximab antibodies in inflammatory bowel disease. J Gastroenterol 2012;47:136-143.PMID: 21953314.


9. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a CrohnŌĆÖs disease activity index. National Cooperative CrohnŌĆÖs Disease Study. Gastroenterology 1976;70:439-444.PMID: 1248701.


10. Sou S, Matsui T, Yao T, et al. Clinical and endoscopic healing after infliximab treatment in patients with CrohnŌĆÖs disease. Dig Endosc 2006;18:29-33.

11. Beppu T, Ono Y, Matsui T, et al. Mucosal healing of ileal lesions is associated with long-term clinical remission after infliximab maintenance treatment in patients with CrohnŌĆÖs disease. Dig Endosc 2015;27:73-81.PMID: 24833527.


12. Imaeda H, Bamba S, Takahashi K, et al. Relationship between serum infliximab trough levels and endoscopic activities in patients with CrohnŌĆÖs disease under scheduled maintenance treatment. J Gastroenterol 2014;49:674-682.PMID: 23666424.


13. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in CrohnŌĆÖs disease. Inflamm Bowel Dis 2009;15:1295-1301.PMID: 19340881.


14. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002;359:1541-1549.PMID: 12047962.


15. Ungar B, Levy I, Yavne Y, et al. Optimizing anti-TNF-alpha therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2016;14:550-557.PMID: 26538204.


16. Roblin X, Marotte H, Leclerc M, et al. Combination of C-reactive protein, infliximab trough levels, and stable but not transient antibodies to infliximab are associated with loss of response to infliximab in inflammatory bowel disease. J Crohns Colitis 2015;9:525-531.PMID: 25895875.



17. Vande Casteele N, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol 2013;108:962-971.PMID: 23419382.



18. Ungar B, Chowers Y, Yavzori M, et al. The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut 2014;63:1258-1264.PMID: 24041539.


Fig.┬Ā1
Overview of study protocol, subject selection and inclusion criteria in Study. The inclusion criteria in Study 1 and Study 2 were: (1) efficacy of initial infusion of infliximab (IFX) was response, were undergoing maintenance therapy; (2) provided informed consent to blood sampling to measure IFX blood concentrations and to endoscopy; (3) their course could be followed up sufficiently; (4) their CDAI could be measured; and (5) were able to undergo colonoscopy or double-balloon enteroscopy within 2 months before or after the date of IFX blood concentration measurement. Exclusion criteria were: (1) continuous administration of IFX for Ōēż14 weeks or Ōēź5 years; (2) a stoma; or (3) not obtaining consent. A total of 72 patients were excluded. In the study design in Study 1, the first assay (assay A and B) was performed with patients divided into loss of response (LOR) group and remission group. Assay A and clinical symptoms in the antibodies to IFX (ATI)-positive patents in the remission group were checked after 1 year. In Study 2, endoscopic examination and assay A were performed after enrollment.
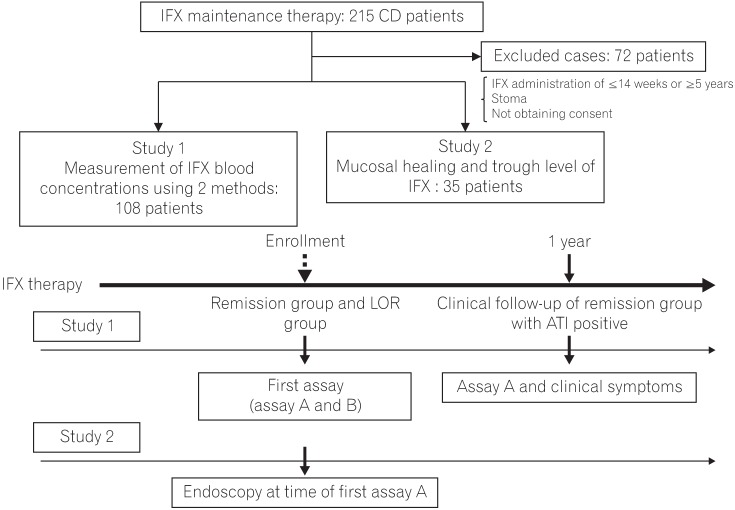
Fig.┬Ā2
Receiver operating characteristic (ROC) curve and cutoff value of infliximab (IFX) trough level and antibodies to IFX (ATI) by assay B (Study 1). (A) ROC curve-cutoff value of the IFX trough level in CD was calculated, as was association between IFX trough level and loss of response, with corresponding sensitivity and specificity for CD. Cutoff value, 2.6 ┬Ąg/mL; area under the ROC curve (AUROC), 77.8. (B) ROC curve-cutoff value of ATI in CD was calculated, as was association between ATI and loss of response, with corresponding sensitivity and specificity for CD. Cutoff value, 4.9 ┬Ąg/mL; AUROC, 67.9.
Fig.┬Ā3
Antibodies to infliximab (ATI)-positive rates in loss of response (LOR) group and in remission group. ŌĆ£ATI positiveŌĆØ was defined as ATI positivity in assay A and ATI of Ōēź4.9 ┬Ąg/mL in assay B. Assay B was used to measure the percentage of patients with ATI in both the LOR and remission groups. ATI-positive rates with assays A and B, respectively, are 9.1% vs. 65.5% in the LOR group, and 3.8% vs. 32.1% in the remission group. Comparison of ATI-positive rates in the LOR and remission groups shows P=0.4379 with assay A, compared to P=0.0006 with assay B.

Fig.┬Ā4
A) Comparison of infliximab (IFX) trough levels between mucosal healing (MH) group and non-MH (nMH) group with lesions of small intestine (Study 2). MH occurred in 10 patients, and there were 21 patients in the nMH group. TLI (trough levels of infliximab; median values) in the MH and nMH groups were 2.5 ┬Ąg/mL vs. 1.8 ┬Ąg/mL, respectively. TLI in the MH and nMH groups showed no significant difference (P=0.380). Number of patients positive for antibodies to IFX (ATI) with assay A: 1 patient (10.0%) in the MH group and 3 patients (14.3%) in the nMH group. (B) Comparison of IFX trough levels between MH group and nMH group with lesions of large intestine (Study 2). There were 13 patients with MH and 8 patients in the nMH group. TLI (median values) in the MH and nMH groups were 2.7 ┬Ąg/mL vs. 0.5 ┬Ąg/mL, respectively. Comparison of TLI between the 2 groups showed a significant difference (P=0.032). Number of patients positive for ATI with Assay A: 0 patients (0.0%) in the MH group and 2 patients (25.0%) in the nMH group.
Table┬Ā1
Characteristics of CD Patients with Infliximab Maintenance Treatment (Study 1)
Table┬Ā2
Characteristics of Patients Who Underwent Endoscopy in Study 2
Table┬Ā3
Comparison of TLI and ATI Studies Using ROC Curve Analysis
| Author | Year | No. | LOR vs. remission | TLI | AUC | Se/Sp (%) | ATI level | AUC | Se/Sp (%) |
|---|---|---|---|---|---|---|---|---|---|
| Steenholdt et al.4 | 2011 | 85 | 26 vs. 59 | 0.50 | 0.930 | 86.0/85.0 | 10.00 U/mL | 0.890 | 81.0/90.0 |
| Vande Casteele et al.5 | 2015 | 483 | NA | 2.79 | 0.681 | 52.5/77.6 | 3.15 U/mL | 0.632 | 38.0/87.4 |
| Present study | 2015 | 108 | 55 vs. 53 | 2.60 | 0.778 | 70.9/79.2 | 4.90 ╬╝g/mL | 0.679 | 65.5/67.9 |




 .
.

