The development and success of anti-tumor necrosis factor-α (anti-TNF-α) monoclonal antibodies (mAbs) have dramatically changed the therapeutic strategy of IBD and contributed to improvements in patients' quality of life.
1 Many clinical observations have confirmed that anti-TNF-α mAbs are efficacious for the treatment of CD. Unfortunately, however, we are faced with a new complication: loss of response (LOR). In Japan at the time of this study, an infliximab (IFX) dose escalation regimen to 10 mg/kg was only allowed in patients who developed LOR during scheduled maintenance therapy with IFX (5 mg/kg every 8 weeks) according to the results of a preapproval clinical trial. However, we often encounter patients who cannot gain adequate control with increasing doses of 10 mg/kg of IFX in daily clinical practice. In this open-labeled prospective study (UMIN registration No. 000010058), patients with non-colonic CD who had developed LOR to scheduled administration of IFX (5 mg/kg every 8 weeks) were randomly assigned to IFX dose escalation (10 mg/kg every 8 weeks) with (combination group) or without (monotherapy group) an elemental diet (ED, 900-1200 kcal/day) for 56 weeks (
Table 1,
Supplementary Figs. 1 and
2). The study was approved by the Institutional Review Board of Keio University Hospital (IRB No. 20120438) and performed in accordance with the principles of the Declaration of Helsinki. The informed consent was written informed consents were obtained. The primary endpoint was the retention rate of IFX dose escalation therapy at week 56. Safety, the CDAI, and the serum albumin and CRP levels at each observation point were analyzed. Intermediate analysis was performed on 15 patients enrolled by 31 March 2015. At week 16, 8 of 11 patients in the combination group and 0 of 4 patients in the monotherapy group showed a CDAI reduction from baseline (ΔCDAI) of ≥50. The intermediate analysis showed the benefits of combination therapy and disadvantage of monotherapy, and the study was stopped. Subsequent analysis showed that the proportion of patients with ΔCDAI of ≥50 (
Fig. 1A) and clinical remission (
Fig. 1B) at each observation point tended to be higher in the combination group. Compared with the monotherapy group, the combination group showed a tendency toward a superior persistence rate of IFX (10 mg/kg every 8 weeks) at week 56 as the primary endpoint in both the per-protocol set analysis (
P=0.1066) and full analysis set analysis (
P=0.1629) (
Fig. 2). No serious adverse events were observed in either group (
Supplementary Table 1). This appears to be the first clinical trial to show the usefulness of combination therapy of enteral nutrition (EN) therapy with biologics for refractory CD.
The effectiveness of EN therapy in patients with CD, especially for the maintenance of clinical remission, has been previously reported.
2,3,4 Several recent reports have described combination therapy involving anti-TNF-α mAb therapy with EN therapy. Hirai et al.
5 examined 102 patients with CD who were treated with IFX. The cumulative remission rate was significantly higher in the combination therapy group (IFX+EN) than in the non-EN group. Kamata et al.
6 reported that concomitant ED therapy (≥900 kcal/day) with IFX scheduled maintenance therapy prevented LOR. Sazuka et al.
7 also reported the benefit of concomitant use of EN therapy (≥600 kcal/day) with IFX scheduled maintenance therapy. Sugita et al.
8 reported that ED therapy administered concomitantly with adalimumab reduced LOR to adalimumab in IFX-intolerant or IFX-refractory patients. Nguyen et al.
9 performed a meta-analysis based on these reports and concluded that in patients with moderate to severe CD undergoing IFX therapy, combined EN therapy of ≥600 kcal/day affected the increase in the remission maintenance rate. In contrast, Yamamoto et al.
10 reported the results of a prospective study showing that concomitant EN during IFX maintenance therapy did not show a beneficial effect in the maintenance rate of clinical remission in patients with CD. However, in the EN combination group, the CDAI tended to be lower at each observation time point than in the IFX alone group. In the study by Yamamoto et al.,
10 the patients with CD had been newly introduced to IFX. These findings may suggest that IFX and EN combination therapy should be considered in only selected patients, including those with suspected LOR to IFX.
There are several limitations in this study. First, we discontinued this study after obtaining the results of the interim analysis, which actually resulted in statistical underpowering of this investigation. Second, the protocol of this study did not include evaluation of endoscopic activity. Third, maintenance of the relatively high acceptance rate of taking ED in the combination group was influenced by the enrollment of only patients who had appropriate adherence to ED, as judged by the acceptability test before enrollment and the patients' history. Therefore, in daily clinical practice, adherence to oral ED administration is expected to be lower. Improvement of adherence to therapy is an important point when using ED to treat CD.
Fig. 1
Percentage of patients with a decrease in CDAI from weeks 8 to 56. (A) The percentage of patients who satisfied the ΔCDAI of ≥50 from weeks 8 to 56 of treatment is shown. Proportions of patients were compared between the groups by Fisher's exact test. Statistical analyses were performed at a two-sided significance level of 0.05. (B) The percentage of patients who achieved clinical remission, as indicated by a CDAI of <150, from weeks 8 to 56 of treatment is shown. Proportions of patients were compared between the groups by Fisher's exact test. Statistical analyses were performed at a two-sided significance level of 0.05. aSignificant difference. ED, elemental diet; IFX, infliximab.
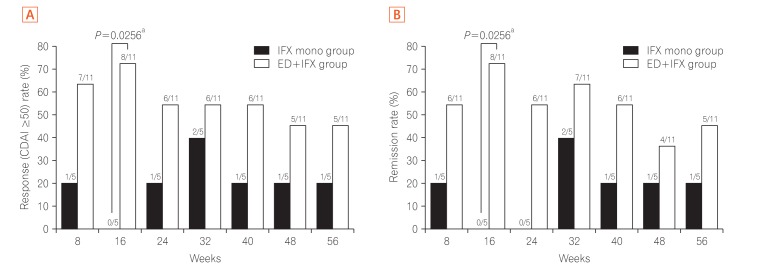
Fig. 2
Cumulative rate of successful completion of the scheduled maintenance treatment with IFX infusion at 10 mg/kg every 8 weeks until week 56. (A) The scheduled maintenance treatment continuation rate curve was estimated by the Kaplan-Meier method for each group, and the groups were compared by the generalized Wilcoxon test (per-protocol set). (B) The scheduled maintenance treatment continuation rate curve was estimated by the Kaplan-Meier method for each group, and the groups were compared by the generalized Wilcoxon test (full analysis set). IFX, infliximab; ED, elemental diet.
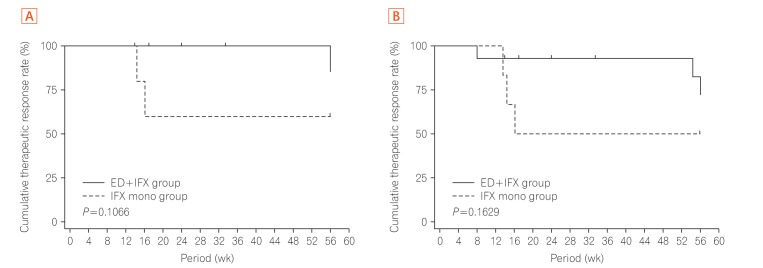
Table 1
Baseline Characteristics
|
IFX mono group (n=6) |
ED+IFX group (n=14) |
P-value |
|
Demographics |
|
|
|
|
Sex (male/female) |
5/1 |
11/3 |
1.0000 |
|
Age (yr) |
35.20±10.11 |
34.80±8.81 |
0.9202 |
|
BMI (kg/m2) |
21.40±4.54 |
21.00±4.32 |
0.9044 |
|
CDAI score |
210.70±17.47 |
211.00±60.78 |
0.1256 |
|
Disease duration |
9.24±7.23 |
11.13±9.04 |
0.9680 |
|
Disease location |
|
|
1.0000 |
|
Ileitis (L1) |
1 (16.7) |
3 (21.4) |
|
|
Ileocolitis (L2) |
5 (83.3) |
11 (78.6) |
|
|
Perianal lesion |
|
|
1.0000 |
|
Yes |
2 (33.3) |
4 (28.6) |
|
|
No |
4 (66.7) |
10 (71.4) |
|
|
Previous surgical resections |
|
|
1.0000 |
|
0 |
3 (50.0) |
6 (42.9) |
|
|
≥1 |
3 (50.0) |
8 (57.1) |
|
|
Current smoking |
1 (16.7) |
2 (14.3) |
1.0000 |
|
Medication at entry |
|
|
|
|
Immunomodulator |
2 (33.3) |
4 (28.6) |
1.0000 |
|
Steroid use |
0 |
0 |
- |
|
5-ASA |
5 (83.3) |
12 (85.7) |
1.0000 |
|
Concomitant medication |
|
|
|
|
Immunomodulator |
2 (33.3) |
5 (35.7) |
1.0000 |
|
Steroid use |
1 (16.7) |
0 |
0.3000 |
|
5-ASA |
5 (83.3) |
12 (85.7) |
1.0000 |
|
CRP (mg/dL) |
0.34±0.26 |
0.74±0.81 |
0.5466 |
|
Serum albumin (g/dL) |
3.88±0.32 |
3.91±0.63 |
0.4268 |








 .
.

