INTRODUCTION
Korean legislation governing the research and development of natural product-derived drugs defines a natural product as that originating from a living organism, including cells or tissue cultures produced from animals and plants. Natural products have been used as drugs for millennia in diverse Asian medical traditions: Korean Hanyak, Traditional Chinese Medicine, Japanese Kampo medicine, Indian Ayurvedic medicine, and Indonesian Jamu medicine. Early in their use, these agents were administered as teas, hot packs, powders, or liquids.
Studies focused on the functions, chemical structures, activities, effective production methods, and usage of substances developed from natural products began around the end of the 18th century. The first scientific study of natural products described extraction of tartaric acid from grapes, citric acid from lemons, malic acid from apples, lactic acid from milk, and uric acid from urine.1 In 1805, a 21-year-old German pharmacist, Friedrich Serturner, separated morphine from opium, which represented the first pharmacologically active plant-derived compound.2 Since then, alkaloids, terpenoids, and glycosides have been isolated, and their chemical structures determined. Early in the 20th century, a microelement analysis method was developed that enabled the production of natural organic compounds from a small amount of raw material. The Russian botanist Mikhail Tswet introduced column chromatography for the separation of substances, which spurred the discovery of numerous biologically active substances, ushering in an era of natural product chemistry.
Some of the most important medications developed in the 20th century were synthesized steroids, whose raw materials came from plants. Oral contraceptive pills and adrenocortical hormones are synthesized from the raw materials of Dioscorea macrostachya and the alkaloid diosgenin. Once the functionality of a natural product is confirmed, it may be used in a wide variety of products, including pesticides, functional foods, cosmetics, and drugs. Natural product-derived substances can be profitable; Dong-A Pharmaceutical has earned approximately 400 billion won since the 2002 approval of Stillen, the best-selling natural-product-derived drug in Korea, whose raw material is Moxa extract.
In the early 1940s, approximately 90% of drugs were derived from natural products. From 1940 to the mid-1980s, most new drugs were organically synthesized. Since that time, approximately 60% of new drugs have originated from natural products.3 In particular, anticancer agents, as well as medications for pain, and disorders of the nervous system, metabolism, and the circulatory system are typically derived from natural products.
NATURAL PRODUCTS UNDER INVESTIGATION FOR THE TREATMENT OF IBDs
1. Aloe Vera
Aloe vera (aloe) is a tropical plant used globally in traditional medicine.6 Its efficacy has been confirmed for the treatment of UC. Aloe gel is the mucous extracted from the leaves of Aloe vera. Because aloe juice has anti-inflammatory effects, some doctors have used it to treat UC patients.7 A small-scale, double-blind, randomized controlled trial was conducted to investigate the efficacy of aloe gel for the treatment of mild-to-moderate UC, which involved oral intake of 100 mg of aloe gel for 4 weeks (30 participants) or oral intake of a placebo substance (14 participants). Nine (30%) of the 30 participants who ingested aloe gel experienced a clinical remission, while 11 (37%) showed improvement. In the control group, 1 participant (7%) experienced clinical remission, while 2 (14%) showed improvement.8 Nevertheless, this study had a small sample size, and, compared with other studies, the control group's response to the placebo substance was low. Although the exact mechanism of aloe is not fully understood, an in vitro study found that aloe gel reduced secretion of prostaglandin E2 and interleukin-18 (IL-18) in the colon mucosa, which suggested that the gel had anti-inflammatory and anti-microbial effects.8 In an in vivo study, aloe extract reduced tumor necrosis factor-α (TNF-α) levels and the expression of IL-1β mRNA, which were indicative of anti-inflammatory effects.
2. Boswellia Serrata
Boswellia serrata is an Indian mastic tree, and the resin collected from its stem has been used in traditional medicine. Boswellic acid, which is thought to contain most of the pharmacologically active ingredients present in the resin, is the substance extracted from B. serrata. In vitro and in vivo, boswellic acid selectively blocked 5-lipoxygenase, indicating an anti-inflammatory effect.9 Because IBD is associated with increased leukotriene function, the effect of boswellic acid on UC was confirmed,10 and its efficacy in reducing edema and inflammation in the small intestine was also confirmed.11 In a study using B. serrata in 30 chronic UC patients, its efficacy on UC with minimal side effects was confirmed.10 In animal experiments, B. serrata was confirmed to be effective for the treatment of CD, UC, and ileitis.12
3. Licorice
Licorice root has been used in Chinese and Korean traditional medicine. Glycyrrhizin (chemical formula, C42H62O16) is extracted from licorice and used as an artificial sweetner. In a study conducted on refined glycyrrhizin, diammonium glycyrrhizinate was confirmed to be useful for the treatment of UC.13 Diammonium glycyrrhizinate was confirmed to reduce inflammation by reducing levels of nuclear factor-kappa B (NF-κB), TNF-α, and intercellular adhesion molecule-1 in the intestinal mucosa of mouse.14
4. Slippery Elm (Ulmus Rubra)
Slippery Elm has been used traditionally to treat coughing, diarrhea, and gastrointestinal tract diseases by Native Americans. Recently, the bark of Slippery Elm was suggested to be effective for treating IBD patients owing to its antioxidant effects.15,16 However, further studies are needed to confirm its efficacy.
5. Tormentil (Potentilla Erecta)
Tormentil (Potentilla erecta) is a perennial plant belonging to the order Rosales, family Rosaceae. Its roots are rich in tannins, which have anti-inflammatory effects. In a small-scale study, tormentil was confirmed to be effective against infectious diarrhea and for the prevention of travelers' diarrhea.17 Another study confirmed its antioxidant effects in IBD patients.7 In yet another study, the administration of various doses of tormentil extract to 16 patients resulted in clinical improvement during the administration period, but the clinical activity index increased upon completion of administration.18
6. Wheat Grass (Triticum Aestivum)
Wheat juice has been used for more than 30 years as a treatment for inflammatory diseases and various gastrointestinal tract diseases, including cancer, but no clinical data are available. However, the intake of fresh wheat juice on an empty stomach has been shown to be an effective treatment. In a randomized controlled trial with a small sample size conducted in 2002, wheat juice produced a 70% improvement in distal UC patients without serious side effects.19
7. Curcumin
Curcumin is a polyphenol extracted from the root of the East Indian rhizomatous perennial Curcuma longa (Zingiberaceae). The rhizomes of C. longa are used to produce turmeric, which is an ingredient in curries and other Indian dishes, and is also used as a yellow pigment. Curcumin, a primary component of turmeric, has been shown to have antioxidant, anti-inflammatory, and anti-cancer effects, and to be effective against enteritis in vitro and in vivo.20 Curcumin reduces local production of cytokines and chemokines, and inhibits infiltration of neutrophils into the mucous membrane. It also controls inflammation by regulating genes associated with oxidative stress and fiberization. The anti-inflammatory effect of curcumin is mediated by interference with arachidonic acid synthesis and blocking the NF-κB activity that is associated with the synthesis of cyclooxygenase 2, 5-lipoxygenase, and inducible nitric oxide.21 There have been 2 human studies on IBD, including a pilot study conducted on 5 UC and 5 CD patients.22 In this study, 5 UC patients and 4 CD patients showed improvement. In a multi-institutional, randomized, double-blind study targeting 89 non-active UC patients, 45 patients took 1 g of curcumin every day in addition to sulfasalazine or mesalazine, while 44 patients took a placebo substance in addition to sulfasalazine or mesalazine.23 After 6 months, 2 (4.65%) of 43 patients who received curcumin (excluding 2 patients who did not follow the protocol) experienced recurrence, compared to 8 (20.51%) of 39 patients who received a placebo substance. In addition, the curcumin-treated group showed better average clinical activity indices and endoscopic indices than the placebo group.
8. Coriolus Versicolor
Coriolus versicolor is also known as the cloud mushroom, and its extracted polysaccharide constituent has anti-cancer and anti-IBD properties.24 In a dextran sulfate sodium (DSS)-induced colitis model, C. versicolor extract reduced the expression of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6. Furthermore, it reduced expression of signal transducers and activators of transcription 1 (STAT1) and STAT6 molecules, which reduced expression of interferon-gamma (IFN-γ) and IL-4.
9. Inonotus Obliquus (Chaga)
Chaga is a parasitic mushroom that grows on birch trees in cold regions. Since the 16th century, Chaga has been used in Russia and Eastern Europe as a folk remedy for various diseases with few, if any, side effects. In Northern and Eastern Russia, Chaga solution has been used for the prevention and treatment of gastrointestinal tract diseases.25 Chaga contains steroids, including polyphenolics, triterpenoids, lanosterol, inotodiol, trametenolic acids, and ergosterol peroxides.26 A recent study reported that I. obliquus extracts inhibited colitis in DSS-induced BALB/c mice, and reduced expression of TNF-α, IL-4, STAT1, and STAT6.27
10. Prunus Mume
Prunus mume is a deciduous tree of the family Rosaceae, and its fruit has been used as a Korean folk remedy for fever, coughing, and intestinal diseases.28 The smoked and dried immature fruit of P. mume, which are called "Omae," have been used in traditional medicine. Omae extract was approved as a drug in 2001 by the China Food and Drug Administration (Approval No. Z11021100), and has been reported to improve colitis symptoms by reversing large intestine damage and abnormally increased cytokine secretion.29 A study conducted in Korea reported that P. mume mixture reduced the expression of TNF-α, cyclooxygenase-2 (COX-2), IL-4, STAT6, INF-γ, and STAT1 in a DSS mouse model.30
11. Gardenia Jasminoides
Gardenia jasminoides has been used in Asia as a folk remedy, and its extract has anti-oxidant effects, such as the removal of various radicals. An in vitro study confirmed this anti-oxidant capability, as well as nitrite elimination, linoleic acid oxidation-blocking ability, and activities similar to superoxide dismutase and catalase.31 In a DSS-induced mouse model of enteritis, glycoprotein separated from the fruits of G. jasminoides inhibited myeloperoxidase activity and reduced the reactive substance concentration of thiobarbituric acid and the production of nitric oxide. Over-generation of inducible nitric oxide synthase (iNOS), COX-2, and NF-kB were also blocked by administration of the glycoprotein isolated from G. jasminoides.32
12. Ginger
Ginger is used as a spice and has anti-oxidant effects. In a mouse model of 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced enteritis, ginger and its constituent zingerone improved enteritis symptoms. Ginger and zingerone inhibited NF-kB activity and reduced IL-1β protein concentration in the large intestine.33
13. Garcinia Cambogia
Garcinia cambogia (also known as Malabar tamarind) is a plant native to Southeast Asia. Its fruit extract has various pharmacological effects, including anti-ulcer activity. In a TNBS-induced mouse model of enteritis, G. cambogia extract produced anti-inflammatory effects; it inhibited expression of myeloperoxidase, COX-2, and iNOS, and reduced concentrations of prostaglandin E2 and IL-1β.34
14. Green Tea
Green tea is made from the leaves of Camellia sinensis. Polyphenols in green tea, such as catechin, act as antioxidants that prevent the activity and transcription of NF-kB. Catechin has been shown to reduce concentrations of inflammation mediators in vivo and in vitro.35 Epigallocatechin-3-gallate (EGCG), a gallic acid ester of catechin, blocks the production of TNF-α, IFN-γ, NF-kB, and p65, which reduces inflammation in the intestinal mucous membrane.36,37 In addition, EGCG improves severe enteritis symptoms and reduces myeloperoxidase activity.38
15. Flavonoids
Flavonoids can act as antioxidants that prevent production of free radicals. Rutin (3-O-rhamnosyl-glucosyl-quercetin) is a common flavonoid found in buckwheat, parsley, and apricot. Rutin has been shown to be effective in the TNBS-induced colitis and acetic acid-induced colitis models in mice.39,40 Rutin was also effective in a DSS-induced mouse model, and has been confirmed to produce anti-inflammatory effects through inhibition of IL-1β and IL-6 gene expression. Rutin has been confirmed to possess preventive and treatment effects in intestinal diseases. Low-dose Rutin improves enteritis symptoms through its regulation of pro-inflammatory mediator genes, such as IL-1β, IL-6, granulocyte-macrophage colony-stimulating factor, and iNOS.41
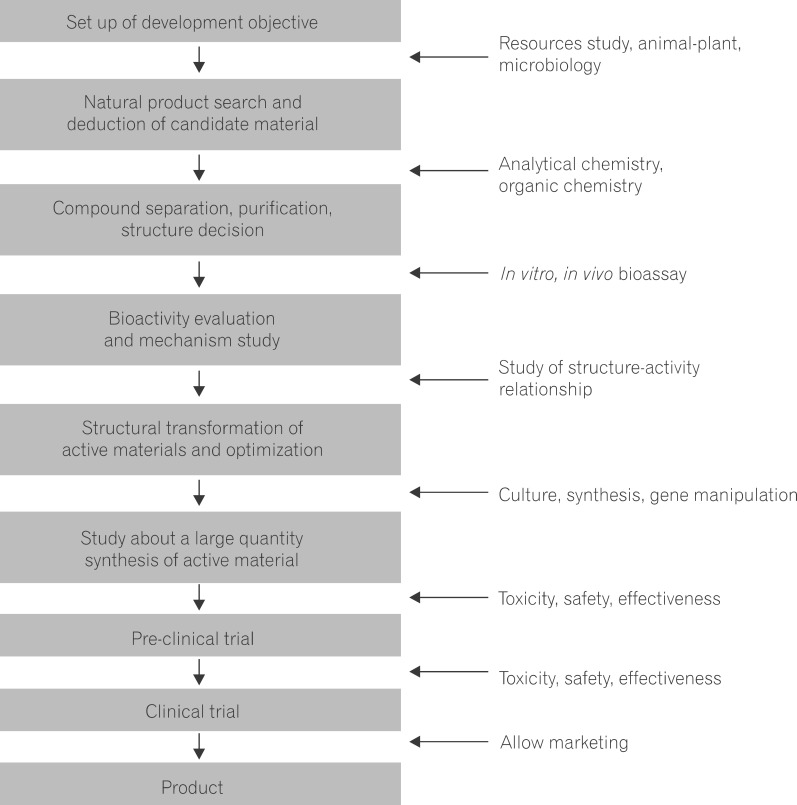
16. Drug Development using Natural Products (Fig. 1)
The development of drugs based on natural products is usually initiated by botanists, folk botanists, folk pharmacologists, and botanical ecologists, who have collected data on plants containing compounds that are efficacious folk remedies.42,43 Because basic clinical and toxicological information, which is necessary for the development of natural product-derived drugs, can be obtained from accounts of personal experience and books on traditional medicine, the time needed to select new drug candidates and collect relevant information may be shortened, compared with that of traditional laboratory-based library screening methods for drug discovery and development.44
Botanical chemists prepare plant extracts that are biologically screened. Through quantitative analyses, the activities and features of compounds are confirmed, and a separation process is initiated. Using molecular biology, physiologically appropriate molecular goals are determined.45,46 Because natural products under study have often been used as folk remedies for centuries or millennia, their efficacy and toxicity have often been verified. Accordingly, new drug development may be completed in a short period of time at a comparatively low cost. However, due to the unique characteristics of natural products, it is difficult to collect and standardize data on their mechanisms and pharmacological effects, and a high level of effort may be required to overcome these difficulties.47
Even after a drug is successfully developed, side-effect prevention and quality control are challenging tasks, because natural products contain more ingredients than synthetic drugs, which complicate studies of their mechanisms of action. Side effects of drugs made from natural products are often reported, and in these cases toxicity may have resulted from the natural products themselves, heavy metal contamination, or pesticides/herbicides used during the cultivation process.48 These factors affect the quality of drugs made from natural products, and supervision and management of natural products are necessary to mitigate their influence. The ingredients, dose, and efficacy of a drug isolated from a natural product may vary according to climate, place of origin, soil, cultivation method, time of collection, and processing method. Therefore, the consistent effort of pharmacologists, botanical chemists, and natural product scientists is necessary to improve the quantity and quality of these drugs.49
The development of a new drug usually takes more than 10 years and costs more than $800 million.42 During the development process, time and effort are spent that are not guaranteed to result in a successful drug. In general, for every 5,000 candidate compounds, only 1 compound successfully progresses through clinical trials and earns approval for clinical use.50
CONCLUSIONS
Interest is growing in the development of efficient new drugs derived from natural sources for the treatment of IBDs, such as UC and CD. As frequently reported in clinical trials, many intestinal disease patients have tried natural products and show great interest in them. This patient preference should encourage the development of new drugs derived from natural products.