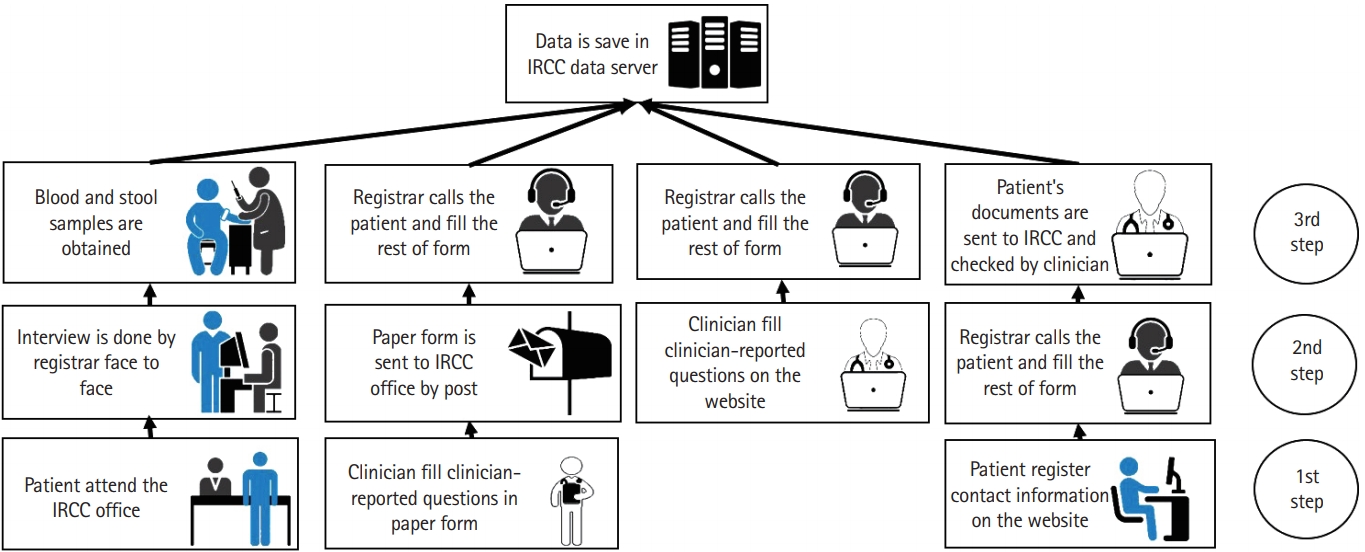
|
 |
- Search
| Intest Res > Volume 17(3); 2019 > Article |
|
Abstract
Background/Aims
Methods
Results
Conclusions
NOTES
FINANCIAL SUPPORT
This work was supported by Deputy of Research of the Ministry of Health and Medical Education, and Iranian Association of Gastroenterology and Hepatology.
AUTHOR CONTRIBUTION
Study design, data analysis, writing the first draft, finalizing the manuscript: Malekzadeh MM. Study design and conduct, data acquisition, revising the manuscript and confirm the final version of manuscript: Sima A, Vahedi H. Study design, revising the manuscript and confirm the final version of manuscript: Alatab S, Zendedel K. Study design, data acquisition, revising the manuscript and confirm the final version of manuscript: Sadeghi A, Malekzadeh R. Data acquisition, revising the manuscript and confirm the final version of manuscript: Daryani NE, Adibi P, Maleki I, Vossoughinia H, Fakheri H, Yazdanbod A, Taghavi SA, Aghazadeh R, Somi MH. Approval of final manuscript: all authors.
ACKNOWLEDGEMENTS
SUPPLEMENTARY MATERIAL
Fig. 2.
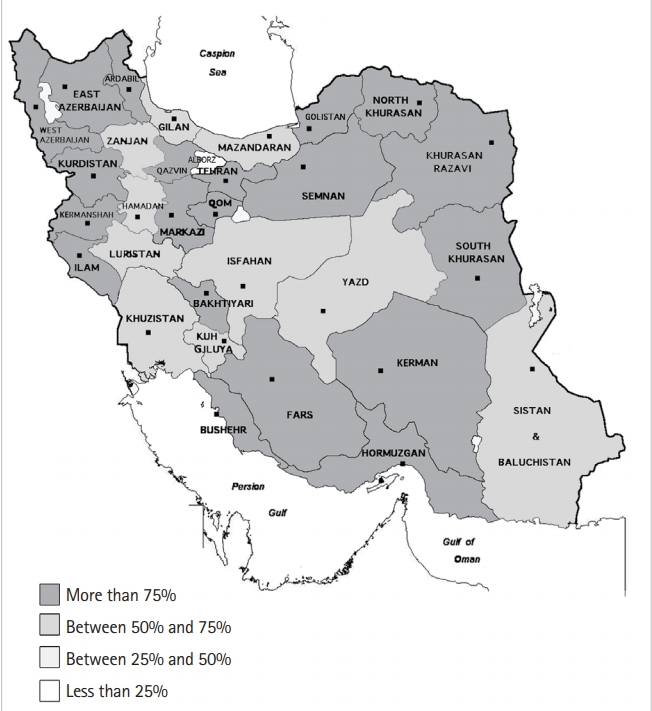
Fig. 4.
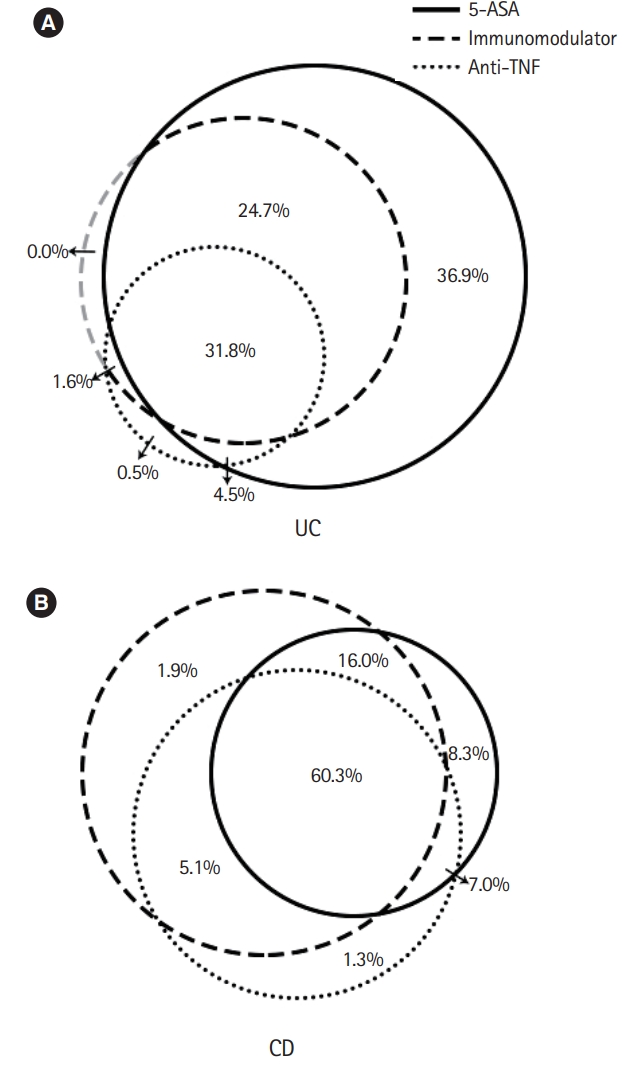
Table 1.
Table 2.
| Characteristic | Value |
|---|---|
| Sex | |
| Male | 312 (56.42) |
| Female | 241 (43.58) |
| Age group (yr) | |
| 0-19 | 21 (3.80) |
| 20-29 | 106 (19.17) |
| 30-39 | 206 (37.25) |
| 40-49 | 120 (21.70) |
| 50-59 | 61 (11.03) |
| 60-69 | 27 (4.88) |
| ≥70 | 12 (2.17) |
| Education | |
| Illiterate | 6 (1.08) |
| Primary school | 20 (3.62) |
| Middle school | 58 (10.49) |
| High school | 166 (30.02) |
| Associate degree | 49 (8.86) |
| Bachelor | 170 (30.74) |
| Master | 65 (11.75) |
| Doctoral | 19 (3.44) |
| Depression | |
| No | 500 (90.42) |
| Yes | 53 (9.58) |
| Appendectomy | |
| No | 495 (89.51) |
| Yes | 58 (10.49) |
| Smoking | |
| Never | 485 (87.7) |
| Current user | 36 (6.51) |
| Past user | 32 (5.79) |
| Age (yr) | |
| UC | 39.51±13.25 |
| CD | 36.10±11.78 |
| Both | 38.58±12.87 |
| Hookah | |
| Never | 509 (92.04) |
| Current user | 32 (5.79) |
| Past user | 12 (2.17) |
| Opium | |
| Never | 540 (97.65) |
| Current user | 9 (1.63) |
| Past user | 4 (0.72) |
| Ethnicity | |
| Fars | 358 (64.74) |
| Turk | 90 (16.27) |
| Lor | 33 (5.97) |
| Kurd | 35 (6.33) |
| Arab | 7 (1.27) |
| Other | 30 (5.42) |
| Family history (1st degree) | |
| UC | 50 (9.04) |
| CD | 15 (2.71) |
| Both | 65 (11.75) |
| Family history (2nd degree) | |
| UC | 29 (5.24) |
| CD | 9 (1.62) |
| Both | 38 (6.87) |
| Disease activity during 2 weeks before enrollmenta | |
| Quiescent | 289 (52.26) |
| Active | 264 (47.74) |
| Disease activity during 6 months before enrollmentb | |
| Quiescent | 341 (61.66) |
| Active | 212 (38.34) |
| Age at diagnosis (yr) | |
| UC | 31.36±12.16 |
| CD | 27.92±11.32 |
| Both | 30.43±11.98 |