 |
 |
- Search
| Intest Res > Volume 18(2); 2020 > Article |
|
Abstract
Background/Aims
Opioid-induced bowel dysfunction includes nausea, vomiting, constipation and abdominal distension. We describe patients presenting with gastrointestinal (GI) ulcers and ulcerated strictures secondary to opioid abuse, an entity not well described in literature.
Methods
This retrospective observational study included patients with opioid abuse gastroenteropathy presenting to Dayanand Medical College and Hospital, Ludhiana, India between January 2013 and December 2018. Opioid abuse gastroenteropathy was defined as gastric or small bowel ulcers and ulcerated strictures in patients abusing opioids, where all other possible etiologies of GI ulcers/strictures were excluded. Clinical, biochemical, endoscopic, radiological and histological parameters as well as response to treatment were assessed.
Results
During the study period, 20 patients (mean age, 38.5┬▒14.2 years; 100% males) were diagnosed to have opioid induced GI ulcers and/or ulcerated strictures. The mean duration of opioid consumption was 6.2┬▒3.4 years. The mean duration of symptoms at presentation was 222.1┬▒392.3 days. Thirteen patients (65%) had gastroduodenal involvement, 6 (30%) had a jejunoileal disease and 1 (5%) had an ileocecal stricture. Two patients (10%) presented with upper GI bleeding, 11 (55%) had features of gastric outlet obstruction and 7 (35%) presented with small bowel obstruction. Abdominal pain and iron deficiency anemia were the most common presentations. Only 1 patient (5%) responded to proton pump inhibitors, 3 (15%) had a lasting response to endoscopic balloon dilatation, while all other (80%) required surgical intervention.
Gastrointestinal (GI) ulcers and ulcerated strictures are a common cause of overt or obscure GI blood loss and intestinal obstruction. The commonest identified causes of these ulcers/strictures are peptic, NSAIDs, corrosive, CD, neoplasms, tuberculosis, Beh├¦etŌĆÖs disease, postsurgical and idiopathic [1,2]. An increasing number of patients presenting with unexplained anemia and/or gastric or small bowel obstruction due to idiopathic ulcers and strictures have been diagnosed in our center lately. These patients have a negative workup for common infective and inflammatory causes and deny history of NSAID intake. However, it was observed that some of these patients had been taking significant amounts of opium in various forms. Opium has been hypothesized to cause ulcero-constrictive disease of small bowel by decreasing intestinal motility, increasing resting muscle tone, increased inflammation and ischemia of small bowel [3]. We hereby report the clinical profile and outcomes of such opioid induced gastric and small bowel ulcers and strictures.
This was a retrospective observational study conducted between January 2013 to December 2018 at Dayanand Medical College and Hospital, a tertiary care hospital in Ludhiana, Punjab, India. The study population included all patients who had gastric or small intestinal ulcers and/or strictures, history of opioid abuse and negative evaluation for other causes. Patients were excluded if they had ulcers/strictures due to peptic causes (including Helicobacter pylori induced ulcers), history of NSAIDs or corrosive intake, biopsy proven CD or tuberculosis, Beh├¦etŌĆÖs disease, neoplasms and postsurgical strictures.
Hospital records of all patients who met the inclusion criteria were reviewed for history, physical examination, radiological and endoscopic evaluation. A particular attention was given to demographics, the duration and type of opium consumed, clinical presentation at the time of diagnosis, hematological and biochemical parameters, radiological findings (site and nature of disease), endoscopic/surgical findings and histology (endoscopic/surgical biopsies, where available). Follow-up of patients by a hospital visit or telephone was attempted for all patients and missing data, if any was completed.
All analyses were performed using SPSS version 21.0 software (IBM Corp., Armonk, NY, USA). Quantitative data were expressed as mean┬▒SD and proportionate data in percentages.
The study was approved by the Institutional Review Board of Dayanand Medical College and Hospital (IRB No. 2020-456) and performed in accordance with the principles of the Declaration of Helsinki. This study is a retrospective study using medical record review and so informed consent was waived.
A total of 20 patients (mean age, 38.5┬▒14.2 years; 100% males) fulfilled the inclusion criteria (Table 1). The mean duration of opioid consumption was 6.2┬▒3.4 years. The commonly abused opioids were dextropropoxyphene (n=10, 50%), loperamide (n=5, 25%), tramadol (n=4, 8%), and opium husk (n=2, 10%). Five patients consumed opioids in more than one form (dextropropoxyphene with tramadol [n=1], dextropropoxyphene with opium husk [n=2], and tramadol with opium husk [n=2]).
The mean duration of symptoms at presentation was 222.1┬▒ 392.3 days. Thirteen patients (65%) had gastroduodenal involvement, 6 (30%) had jejunoileal disease, 1 (5%) had ileocecal involvement and none had colonic involvement. Among those with gastroduodenal involvement, 2 presented with upper GI bleeding, while 11 presented with features of gastric outlet obstruction. All 7 patients with ileal (jejunoileal/ileocecal) disease presented with features of small bowel obstruction. The most common presenting complaints were abdominal pain (n=20, 100%), fatigue (n=20, 100%), vomiting (n=18, 90%), weight loss (n=15, 75%; mean weight loss 12.8┬▒8.4 kg), and anorexia (n=13, 65%). All 20 patients (100%) had iron deficiency anemia (mean hemoglobin, 6.8┬▒2.3 g/dL; ferritin, 23.1┬▒10.8 ng/mL) and hypoalbuminemia (2.3┬▒0.5 g/dL).
CT enterography was done in all patients with ulcerated strictures and it showed multiple, short segment strictures with mildly thick and variably enhancing walls. Proximal to these strictures, either the stomach or the small bowel was dilated (Fig. 1). No significant peri-enteric changes were seen. Two patients with GI bleeding had thickened duodenal walls. Esophagogastroduodenoscopy showed Forrest IB duodenal ulcers in both these patients. One of these could be managed with endotherapy (hemoclipping), while the other one required angioembolization of gastroduodenal artery.
Eleven patients (55%) had pyloric or proximal duodenal ulcerated strictures and presented with gastric outlet obstruction (Fig. 2A and B). Proton pump inhibitors were given to all the patients, but were not effective as sole therapy. Eight of these patients (72.7%) underwent endoscopic balloon dilatation (total 12 sessions, 1 session in 5 patients, 2 sessions in 2 patients and 3 sessions in 1 patient), however, only 3 of these (27.27%) had lasting symptomatic relief. Six patients (3 after failed balloon dilatation and 3 who opted for surgery over endoscopic balloon dilatation) underwent surgery (gastrojejunostomy) for the relief of symptoms.
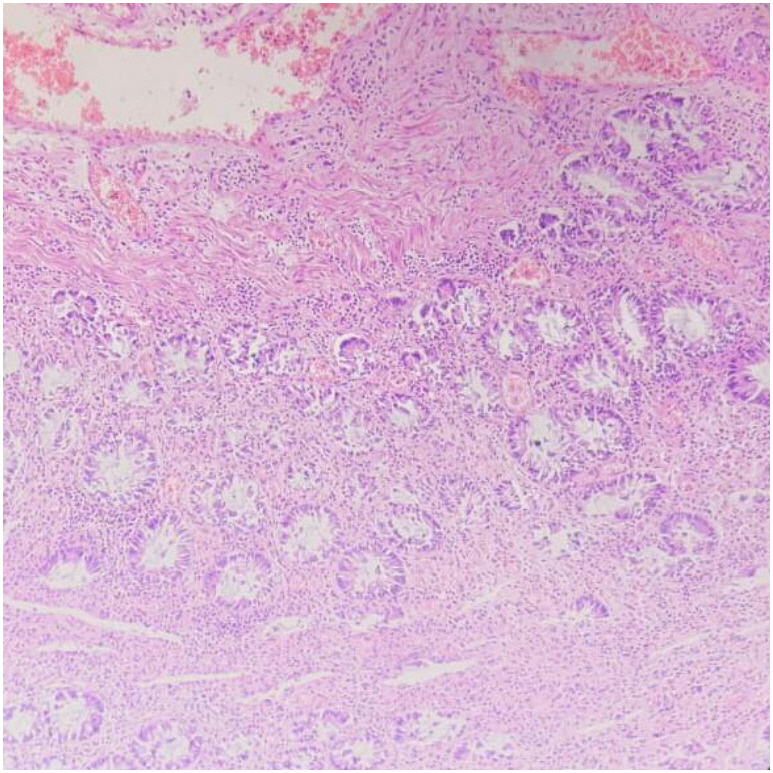
For patients with jejunoileal strictures, the disease site could be reached by antegrade double-balloon enteroscopy in 4 patients (Fig. 2C), and 1 patient had an ileocecal stricture which was diagnosed by colonoscopy (Fig. 2D). Balloon dilatation was also attempted in one patient each with proximal jejunal stricture and ileocecal stricture, but failed to provide a lasting relief. All the patients with ileal involvement hence finally underwent resection of the diseased segment and end to end anastomosis. Histopathological examination of these strictures revealed reactive changes of the epithelium with minimal activity (Fig. 3). Lamina propria had mild degree of chronic inflammatory infiltrate comprised of lymphocytes, plasma cells and few eosinophils. Submucosal regions showed variable degree of fibrosis. No granulomas, necrosis, evidence of ischemic changes, or vascular changes were evident.
Thirteen patients (65%) required blood transfusions and 10 (50%) were given parenteral iron. All patients were counselled for de-addiction. Sixteen patients (80%) came for follow-up, however only 7 patients (35%) adhered to the same. Sustained improvement (>6 months follow-up) in hemoglobin and albumin was noted in 4 out of 18 (22.2%) patients after de-addiction and medical/surgical management.
Opioids are the third most commonly abused drugs in India, after alcohol and tobacco [4,5]. Though GI dysmotility has been described with chronic opioid abuse, GI ulcers/strictures have not been described, except in one series of 6 cases [3]. We also propose the term ŌĆ£opioid abuse gastroenteropathyŌĆØ (akin to NSAID enteropathy) for the ulcers and ulcerated strictures due to opioid abuse, where all other causes have been ruled out. Our patients with opioid abuse gastroenteropathy presented with either gastric outlet or small bowel obstruction. All of them had chronic iron deficiency anemia (secondary to GI blood loss and/or due to bacterial overgrowth due to small bowel strictures), presenting as fatigue and dyspnea on exertion. The main differentials to be considered in such patients are peptic strictures due to H. pylori or NSAIDs, tuberculosis and CD, in addition to post corrosive and postoperative strictures. We observed that opioid abuse most commonly resulted in gastroduodenal, followed by jejunoileal/ileocecal involvement. Patients with tuberculosis and CD, on the other hand, rarely present with isolated gastroduodenal disease. The commonly involved sites in these diseases are terminal ileum and ileocecal valve, both of which were spared in a majority of patients with opioid enteropathy (19/20, 95%) [6]. NSAIDs may present with ulcers and diaphragm like strictures at the same sites as opioids, however, a detailed history can differentiate between the two. Radiologically, CT or magnetic resonance (MR) enterography can help ascertain the site of disease, and detect extraluminal findings, if any that may aid in diagnosis. For example, mural thickening with stratification, comb sign (vascular engorgement of mesentery) and mesenteric fibrofatty proliferation are features which favor CD, while mural thickening with ileocecal involvement, lymphadenopathy with hypodense centers and peripheral enhancement suggesting caseation are suggestive of tuberculosis. Multiphase imaging during CT and MR enterography in NSAID strictures reveal multiple, short segment strictures with minimal enhancement or wall thickening [7]. CT enterography was done in all our patients with opioid enteropathy to ascertain the extent of disease and also to rule out other etiologies of these strictures. We observed that strictures in opioid abusers were similar to NSAID strictures in being multiple, short-segment with minimal wall thickness and variable contrast enhancement. While proton pump inhibitors remain the mainstay of treatment in NSAID induced gastric and duodenal ulcers (except chronic ones which undergo repair with deposition of collagenous scars), these are not helpful in treatment of opioid induced gastroduodenal ulcers and ulcerated strictures. Patients with opioid strictures who failed medical management were subjected to endoscopic balloon dilatation. Of the 10 patients who underwent the same (1 or multiple sessions), only 3 (30%) had a lasting response. Six of the 7 patients who failed balloon dilatation required surgery (gastrojejunostomy). All 7 patients with ileal disease were also subjected to surgery.
Patients with opioid enteropathy showed prominent submucosal changes with reactive changes of the epithelium and mild degree of chronic inflammatory infiltrate (comprised of lymphocytes, plasma cells and few eosinophils) in the lamina propria. This is in contrast to CD where histological evaluation shows transmural inflammation with lymphoid aggregation, infrequent small granulomas which are poorly organized; and tuberculosis where caseation necrosis, large confluent multiple granulomas, with a disproportionate submucosal inflammation are seen [6].
Opioids have been one of the earliest groups of medications used for pain. Chronic opioid use has been increasing worldwide over the past few years, either as prescriptions for cancer and non-cancer pain in Western countries like the USA and Canada [8], or as substance of abuse in Asian countries [9]. In countries like India, restrained use of natural opioids in the form of raw opium or poppy husk is socially accepted in many (especially rural) areas. Raw opium and opioids have been used off label for relief of pain after heavy work, for alleviating anxiety, sedation, control of diarrhea, and in patients with impending stroke. More recently, illicit opioids have replaced raw opium, especially in young males [5,10]. This explains why opioid abuse gastroenteropathy was seen only in young males in our study.
Chronic opioid abuse can negatively affect central nervous system and also the GI system where opioid receptors are present in abundance. This results in inhibition of gastric emptying, increase in sphincter tone, changes in motor patterns, and blockage of peristalsis [11]. Short acting opioids have also been demonstrated to cause a hyperadrenergic state due to opioid withdrawal, which results in increase in blood pressure and heart rate and reduces subendocardial perfusion [12-14]. Opium use has also been shown to be associated with high rates of myocardial fibrosis, perivascular fibrosis, interstitial fibrosis and severe coronary artery disease [15-17]. The pathogenesis of opioid abuse gastroenteropathy can therefore be hypothesized to be due to a combination of (1) a reduction in motility, which results in stasis and prolonged exposure to gastric acid/intestinal contents and (2) intestinal ischemia secondary to the hyperadrenergic and pro-inflammatory state secondary to opioid abuse.
In our experience, opioid abuse gastroenteropathy is a difficult to treat entity. It presents with severe anemia and hypoproteinemia, does not respond to proton pump inhibitors, has a poor response to endoscopic balloon dilatation and most of the patients finally require either a surgical drainage (gastrojejunostomy in gastroduodenal disease) or resection anastomosis (small bowel strictures). In addition, opium deaddiction in itself is challenging [18]. Most of the people with opioid abuse gastroenteropathy have been abusing opioids for several years (6.2┬▒3.4 years in our study) and they require a long term combined psychosocial and pharmacological approach for treatment.
To conclude, all patients who have been abusing opium should be evaluated for opioid abuse gastroenteropathy, as this entity usually presents late, at a stage when medical or endoscopic management usually fails. Early detection of these lesions may prevent formation of resilient strictures and subsequent surgical intervention.
NOTES
FINANCIAL SUPPORT
The authors received no financial support for the research, authorship, and/or publication of this article.
AUTHOR CONTRIBUTION
Conception and design: Mahajan R, Midha V, Sood A. Collection of data: Mahajan R, Singh A, Dhiman P, Sood A. Analysis and interpretation of the data: Mahajan R, Gupta Y, Singh A, Dhiman P, Midha V, Mehta V, Sood A. Drafting of the article: Mahajan R, Gupta Y, Singh A, Midha V, Kakkar C, Narang V, Saggar K, Sood A. Critical revision of the article for important intellectual content: all authors. Final approval of the article: all authors.
Fig.┬Ā1.
CT findings in a patient with opioid abuse gastroenteropathy. (A) Contrast-enhanced CT image showing dilated stomach secondary to narrowing and wall thickening in the D2 segment of duodenum (white arrow) in a 28-year-old patient with a history of long-term opioid abuse who presented with gastric outlet obstruction. (B) The same patient had another stricture at the junction of the D3-D4 segment (black dashed arrow) with upstream duodenal dilatation. S, stomach; D, duodenal.

Fig.┬Ā2.
Endoscopic images of patients with opioid gastroenteropathy showing ulcerated pyloric strictures (A, B); ulcerated small bowel stricture (C); and ileocecal stricture (D).

Fig.┬Ā3.
Histopathological findings in opioid abuse gastroenteropathy. Histopathological examination showed reactive changes of the epithelium with minimal activity, mild degree of chronic inflammatory infiltrate comprised of lymphocytes, plasma cells and few eosinophils in lamina propria and variable degree of submucosal fibrosis (H&E, ├Ś400).
Table┬Ā1.
Clinical Profile and Outcomes of Opioid Gastroenteropathy
REFERENCES
1. Khullar SK, DiSario JA. Gastric outlet obstruction. Gastrointest Endosc Clin N Am 1996;6:585-603.


2. Gill RS, Kaffes AJ. Small bowel stricture characterization and outcomes of dilatation by double-balloon enteroscopy: a single-centre experience. Therap Adv Gastroenterol 2014;7:108-114.



3. Joshi A, Falodia S, Kumar N, Solanki RL. Small intestine strictures in opium addicts: an unrecognized cause of intestinal obstruction. Indian J Gastroenterol 2018;37:169-173.



4. Mohan D, Sundaram KR, Sharma HK. A study of drug abuse in rural areas of Punjab (India). Drug Alcohol Depend 1986;17:57-66.


5. Chaturvedi HK, Mahanta J, Bajpai RC, Pandey A. Correlates of opium use: retrospective analysis of a survey of tribal communities in Arunachal Pradesh, India. BMC Public Health 2013;13:325.




6. Pulimood AB, Amarapurkar DN, Ghoshal U, et al. Differentiation of CrohnŌĆÖs disease from intestinal tuberculosis in India in 2010. World J Gastroenterol 2011;17:433-443.



7. Frye JM, Hansel SL, Dolan SG, et al. NSAID enteropathy: appearance at CT and MR enterography in the age of multi-modality imaging and treatment. Abdom Imaging 2015;40:1011-1025.



8. Berterame S, Erthal J, Thomas J, et al. Use of and barriers to access to opioid analgesics: a worldwide, regional, and national study. Lancet 2016;387:1644-1656.


9. Ray R, Kattimani S, Sharma HK. Opium abuse and its management: global scenario. WHO Web site. https://www.who.int/substance_abuse/activities/opium_abuse_and_its_management.pdf. Accessed August 1, 2019.
10. Farhat S, Hussain SS, Rather YH, Hussain SK. Sociodemographic profile and pattern of opioid abuse among patients presenting to a de-addiction centre in tertiary care hospital of Kashmir. J Basic Clin Pharm 2015;6:94-97.



11. Khansari M, Sohrabi M, Zamani F. The useage of opioids and their adverse effects in gastrointestinal practice: a review. Middle East J Dig Dis 2013;5:5-16.


12. Charney DS, Redmond DE Jr, Galloway MP, et al. Naltrexone precipitated opiate withdrawal in methadone addicted human subjects: evidence for noradrenergic hyperactivity. Life Sci 1984;35:1263-1272.


13. Reece AS, Hulse GK. Impact of lifetime opioid exposure on arterial stiffness and vascular age: cross-sectional and longitudinal studies in men and women. BMJ Open 2014;4:e004521.



14. Darke S, Duflou J, Torok M. The comparative toxicology and major organ pathology of fatal methadone and heroin toxicity cases. Drug Alcohol Depend 2010;106:1-6.


15. Ebdali RT, Tabaee SS, Tabaei S. Cardiovascular complications and related risk factors underlying opium consumption. J Cell Physiol 2019;234:8487-8495.


16. Masoudkabir F, Sarrafzadegan N, Eisenberg MJ. Effects of opium consumption on cardiometabolic diseases. Nat Rev Cardiol 2013;10:733-740.



- TOOLS








