 |
 |
- Search
| Intest Res > Volume 19(3); 2021 > Article |
|
Abstract
Background/Aims
Methods
Results
ADDITIONAL INFORMATION
Funding Source
This research was supported by Medical Research Center Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2017R1A5A2014768).
Conflict of Interest
Park DI and Han DS are editorial board members of the journal but were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author Contribution
Conceptualization: Oh SJ, Lee CK. Data curation: all authors. Formal analysis: Oh SJ, Lee CK. Funding acquisition: Kim HJ. Investigation: Shin GY, Soh H, Lee JG, Im JP, Eun CS, Lee KM, Park DI, Han DS, Kim HJ, Lee CK. Methodology: Lee CK. Writing - original draft: Oh SJ, Lee CK. Writing - review & editing: all authors. Approval of final manuscript: all authors.
Fig.┬Ā1.
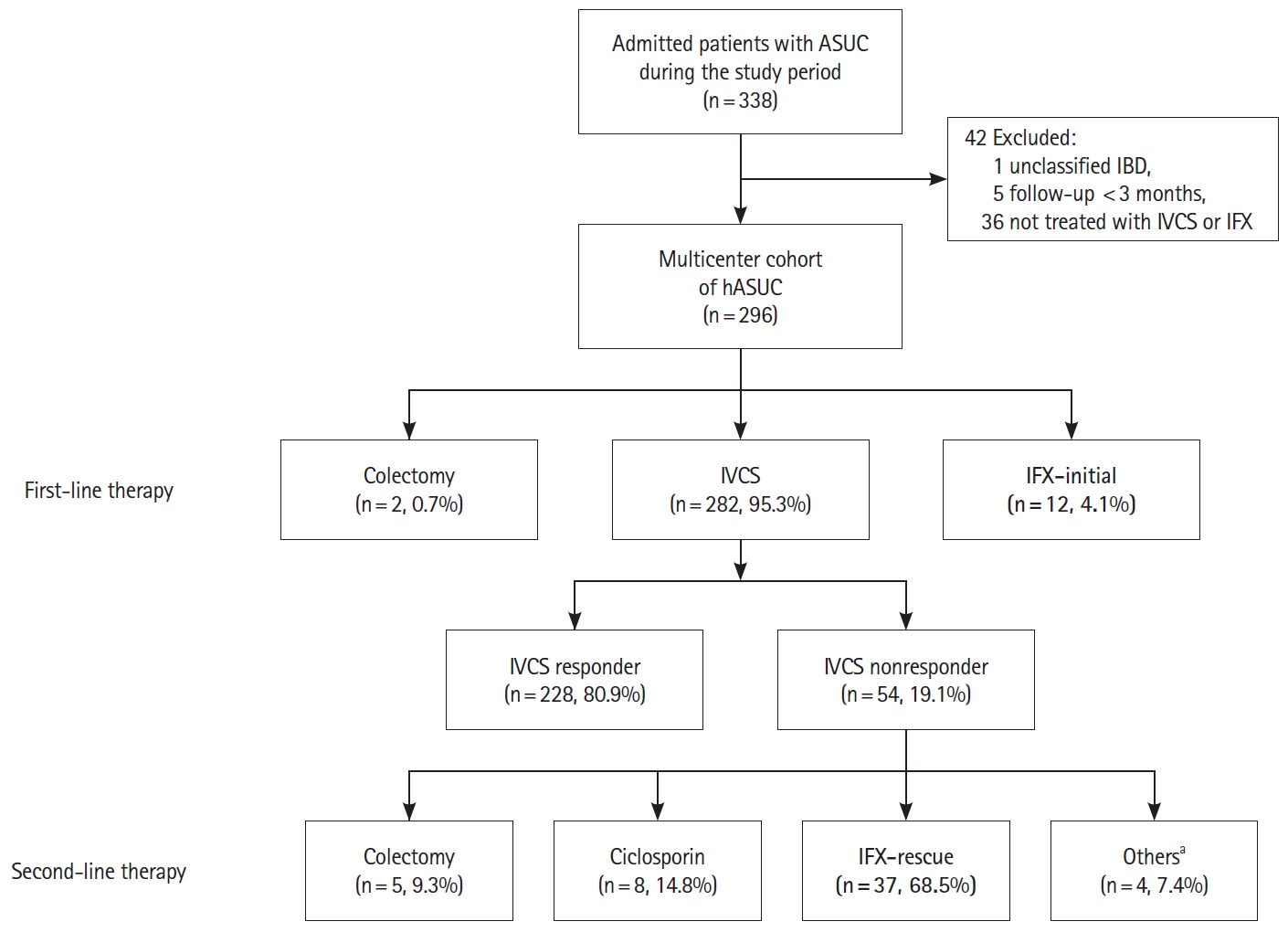
Fig.┬Ā2.
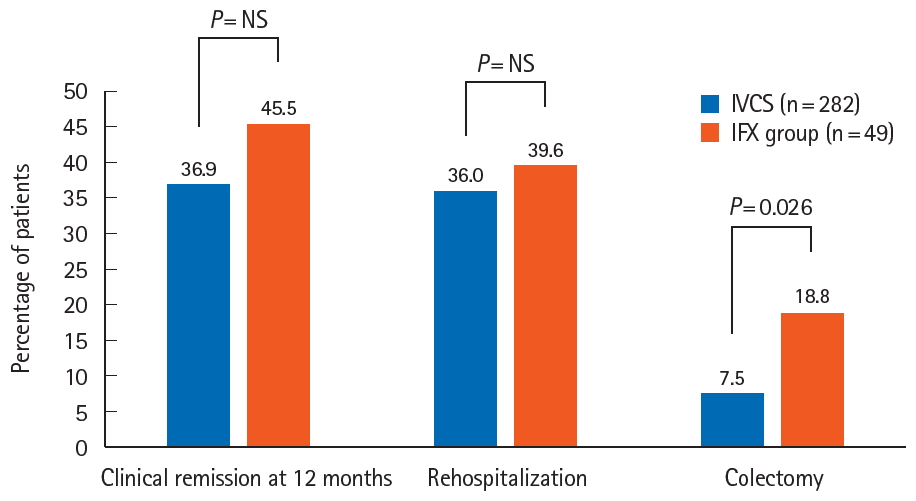
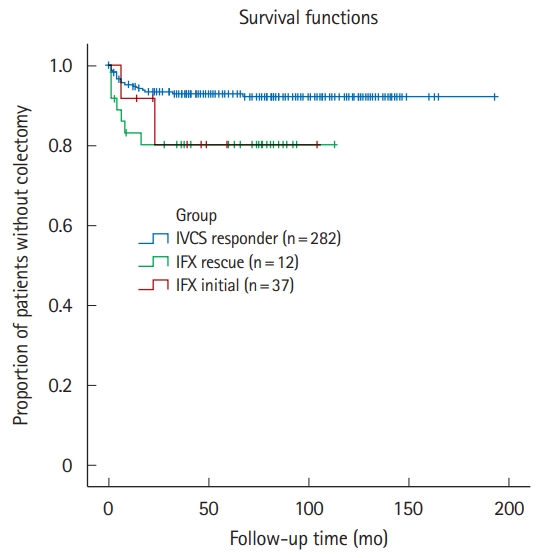
Fig.┬Ā3.
Table┬Ā1.
| Demographic data | IFX group (n=49) | IVCS responder (n=228) | P-value |
|---|---|---|---|
| Age (yr) | 40.8 ┬▒ 17.2 | 44.5 ┬▒ 16.7 | 0.162 |
| Male sex | 28 (57.1) | 121 (53.1) | 0.604a |
| Disease duration (mo) | 48.7 ┬▒ 51.3 | 48.7 ┬▒ 99.9 | 0.993 |
| Smoking habits | 0.881a | ||
| ŌĆāNever smoker | 40 (81.6) | 184 (80.7) | |
| ŌĆāEx- or current smoker | 9 (18.3) | 44 (19.3) | |
| Family history of IBD | 1 (2.0) | 5 (2.2) | 1.000 |
| Disease extent | 0.246a | ||
| ŌĆāE1 | 2 (4.4) | 13 (5.7) | |
| ŌĆāE2 | 27 (58.7) | 103 (45.2) | |
| ŌĆāE3 | 17 (37.0) | 112 (49.1) | |
| Total Mayo score | 10.5 ┬▒ 1.1 | 10.7 ┬▒ 1.3 | 0.323 |
| Laboratory findings | |||
| ŌĆāCRP (mg/dL) | 4.3 ┬▒ 5.0 | 4.9 ┬▒ 5.0 | 0.495 |
| ŌĆāAlbumin (g/dL) | 3.6 ┬▒ 0.7 | 3.4 ┬▒ 0.6 | 0.099 |
| ŌĆāHb (g/dL) | 11.7 ┬▒ 1.9 | 11.5 ┬▒ 2.3 | 0.501 |
| ŌĆāWBC (├Ś 103/╬╝L) | 10.1 ┬▒ 5.7 | 9.8 ┬▒ 3.8 | 0.700 |
| Concomitant treatments | |||
| ŌĆāOral steroids | 31 (63.3) | 79 (34.7) | < 0.001a |
| ŌĆāThiopurines | 21 (42.9) | 28 (12.3) | < 0.001 |
| ŌĆāAnti-TNF agents | 4 (8.2) | 8 (3.5) | 0.235 |
| Hospital stay (day) | 19.8 ┬▒ 14.8 | 14.1 ┬▒ 10.1 | 0.013 |
| CMV colitis | 10 (20.4) | 19 (8.3) | 0.012a |
| C. difficile infection | 2 (4.1) | 6 (2.6) | 0.635 |
Table┬Ā2.
| Variable | IFX-rescuea (n = 37) | IFX-initialb (n = 12) | P-value |
|---|---|---|---|
| Short-termc | |||
| ŌĆāClinical remission | 3 (23.1) | 3 (30.0) | 1.000 |
| ŌĆāRehospitalizatioin | 7 (18.9) | 3 (25.0) | 0.690 |
| ŌĆāColectomy | 3 (8.1) | 0 | 0.566 |
| Long-termd | |||
| ŌĆāClinical remissione | 7 (46.7) | 3 (42.9) | 1.000 |
| ŌĆāRehospitalization | 15 (41.7) | 4 (33.3) | 0.740 |
| ŌĆāColectomy | 7 (18.9) | 2 (16.7) | 1.000 |
Table┬Ā3.
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
| IFX-rescuea | 2.80 | 1.09-7.17 | 0.032 |
| IFX-initialb | 1.81 | 0.06-54.59 | 0.732 |
| Failure of anti-TNFsc | 4.90 | 1.62-14.84 | 0.005 |
| CMV colitis | 6.57 | 2.26-19.08 | < 0.001 |
| C. difficile infection | 4.61 | 1.50-14.23 | 0.008 |
| Duration of IVCS | 1.07 | 1.00-1.15 | 0.040 |








