 |
 |
- Search
| Intest Res > Volume 22(1); 2024 > Article |
|
See Editorial "A novel serum biomarker of endoscopic mucosal healing in inflammatory bowel disease" in Volume 22 on page 3.
Abstract
Background/Aims
The achievement of endoscopic remission is an important therapeutic goal in the treatment of inflammatory bowel diseases (IBD). We aimed to evaluate the role of fecal calprotectin (FCP) and ischemia-modified albumin (IMA) as biomarkers for evaluating IBD disease activity.
Methods
A total of 48 patients with IBD (20 with ulcerative colitis and 28 with Crohnâs disease) were included in this study. FCP and serum C-reactive protein levels, erythrocyte sedimentation rate, and IMA were measured in patients with IBD and compared with endoscopic findings.
Results
Elevated FCP and serum IMA levels were significantly associated with endoscopic non-mucosal healing. The correlation between FCP and IMA was not significant. Analysis of the receiver operating characteristic curve showed that both FCP and IMA had diagnostic value in predicting non-mucosal healing. When the Ln(FCP)+IMA/10 value was calculated using both factors, the predictive value for non-mucosal healing increased; however, no significant difference was observed.
Inflammatory bowel disease (IBD) is a chronic, recurrent, and progressive condition of the gastrointestinal tract that comprises 2 major subtypes: ulcerative colitis (UC) and Crohnâs disease (CD) [1]. The objective assessment of intestinal inflammation is the mainstay in the diagnosis and follow-up of IBD and mucosal healing (MH) the key therapeutic target [1,2]. However, because of the invasiveness and cost of endoscopic examination, as well as its limited capacity, standardized and reliable markers that indicate intestinal inflammation are required [3-5].
Although evaluating disease activity according to clinical symptoms is simple and cost-effective, it is subjective and has a low concordance rate with disease activity on endoscopy, whereas blood systemic inflammatory markers present relatively low sensitivity [6-9]. The Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE-II) group has updated the recommendations for the targeted treatment of IBD, including fecal calprotectin (FCP) as an important marker for disease activity [1]; however, it involves some patient discomfort, as a stool sample is required.
Oxidative stress has been suggested as a potential trigger for IBD [10,11]. Ischemia prompts inflammatory reactions in the intestinal mucosa that release numerous reactive oxygen species (ROS), such as superoxide, hydrogen peroxide, and hydroxyl radicals [12]. Reactive ROS generated during ischemic diseases modify the N-terminal sequence of serum albumin, resulting in ischemia-modified albumin (IMA) formation [13]; such IMA is considered an indirect reagent of oxidative stress, and its increase has been correlated with disease activity in various diseases, including IBD [13-16]. In this study, IBD endoscopic activity and IMA were evaluated and compared with FCP and other inflammatory blood markers.
This study included 48 patients with IBD from March 2020 and December 2022. Patients aged 18 to 80 years with clinically stable disease (Crohnâs Disease Activity Index < 220 for CD patients and a partial Mayo score < 3 for patients with UC) were enrolled [1]. To allow endoscopic evaluation, we attempted selecting patients with CD with colonic disease as study subjects and, if possible, patients were limited to those who had disease only in the terminal ileum, which could be evaluated endoscopically. During the study period, if clinically stable patients with IBD underwent routine colonoscopy, they were screened, and informed consent was obtained. Subsequently, blood was collected for the measurement of IMA, serum C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) within 3 months after endoscopy, and a fecal sample was collected to evaluate FCP.
To compare the endoscopic disease activity, the colonoscopy results were classified into 2 groups: with or without MH. For patients with UC, the Mayo Endoscopic Score was used. A score of 1 was classified as MH and a score ⼠2 was considered nonMH status [17]. For patients with CD, a score between 0 and 2 of the Simple Endoscopic Score for Crohnâs Disease with 0 of the ulceration subscore, was considered endoscopic remission, and the case was assigned to the MH group [18].
Bar-Or et al. [19] developed an assay for assessing IMA based on measuring the degree of its interaction with metal ions, such as cobalt (Co2+); these findings were used to develop the albumin cobalt binding test by Ischemia Technologies Incorporated (Denver, CO, USA), which received U.S. Food and Drug Administration approval in 2003 [12,13]. In principle, a known number of cobalt ions are added to a serum sample and bind to normal albumin, but not IMA. The remaining free cobalt ions react with dithiothreitol to form colored complexes, which can be spectrophotometrically quantified; the IMA concentration is directly proportional to the concentration of the colored complex, and thus to the color intensity.
The FCP concentration was measured using a quantitative enzyme-linked immunosorbent assay (RIDASCREENÂŽ; R-Biopharm AG, Darmstadt, Germany). All fecal samples were processed within 72 hours of collection. Fecal specimens were diluted to 1:2,500. Enzyme-linked immunosorbent assay (ELISA) plates were read using a Spectra Mini Reader. According to the manufacturerâs instructions, samples containing ⼠50 mg/kg feces were considered calprotectin positive [20,21].
For descriptive analysis, categorical variables were expressed as numbers with percentages, and continuous variables were expressed as medians with interquartile ranges (IQR). The t-test was used to evaluate the associations between parametric numerical data, whereas the Mann-Whitney U test was used to explore the associations between nonparametric numerical data. Statistical significance was set at P<0.05. Associations between endoscopic disease activity and FCP concentration, and CRP, ESR, and IMA levels were assessed using univariate and multivariate binary logistic analyses. Variables were selected for inclusion in the multivariate model based on univariate P-values < 0.2. The association between FCP concentration and IMA levels was assessed using the Spearman rank correlation coefficient (r). Receiver operating characteristic (ROC) and area under the curve (AUC) analyses were performed to evaluate the test characteristics of the noninvasive items to predict endoscopically active disease. All analyses were performed using the IBM SPSS software (version 24.0; IBM Corp., Armonk, NY, USA).
All participants were informed about the study and provided written informed consent to participate in it. The study protocol was reviewed and approved by the Institutional Review Board of Ulsan University Hospital (IRB No. 2020-03-017) and conducted in accordance with the Declaration of Helsinki.
Baseline characteristics of the study population are summarized in Table 1. The median age at the time of diagnosis was 29 years; 29 males (60.4%) and 19 females (39.6%) were included in the study. Twenty patients (41.7%) had UC, 5 had left-sided colitis, and 15 had pancolitis; the remaining 28 (58.3%) patients had CD. According to the Montreal classification [22], L1 patients (n = 5) and L3 patients (n = 23) were included in the study. A total of 21 patients (40.8%) were concomitantly treated with biological or small-molecule agents. In cases in which the IMA level was measured, the median value was 72.3 U/mL, and the IQR was 68.7-77.7 U/mL. Regarding FCP, the median value was 165.5 mg/kg (IQR, 87.7-332.1 mg/kg). In the IBD subgroup analysis, no significant difference was identified in IMA levels between the 20 patients with UC and 28 with CD (median [IQR], 72.3 [67.0-77.7] in UC vs. 72.2 [69.3-78.0] in CD; P=0.875]).
A comparison of baseline characteristics according to endoscopic MH is summarized in Table 2. Age, sex, and concomitant medications of the study participants did not present any significant differences according to the MH. When comparing the IMA levels (Fig. 1A), the median value in the non-MH group increased significantly, that is, 69.3 U/mL versus 75.5 U/mL, compared to that in the MH group (P<0.001). For FCP (Fig. 1B), the non-MH group had a significantly higher median value of 82.4 mg/kg versus 292.6 mg/kg compared to the MH group (P<0.001). Other inflammatory markers, such as CRP and ESR, did not present any differences according to endoscopic status in this study. When analyzing the difference in IMA levels according to endoscopic MH by dividing the patients into UC and CD groups, the IMA levels differed signifcantly according to the mucosal state in both IBD groups (Supplementary Table 1, Supplementary Fig. 1).
As a result of the association between biochemical markers and endoscopic MH in the IBD analysis, both higher IMA and higher FCP levels showed a significant association in univariate and multivariate analyses for the prediction of non-MH (IMA, odds ratio = 1.384, P=0.009 and FCP, odds ratio = 1.013, P=0.003, respectively) (Table 3). The correlation between IMA and FCP was not significant (r= -0.017, P=0.912) (Fig. 2). ROC curve analysis revealed that both FCP and IMA possessed significant diagnostic value in predicting non-MH (AUC, 0.867 in FCP vs. 0.801 in IMA) (Fig. 3A). However, no statistically significant differences were identified in the diagnostic values of the 2 test methods (P=0.980). To investigate the role of IMA in complementing FCP, various combinations of FCP and IMA were selected, as shown in Supplementary Fig. 2. When the value of Ln (FCP) + IMA/10 was calculated using both factors, the predictive value for non-MH increased (AUC, 0.944) (Fig. 3B), yet no significant differences were identified when compared with IMA and FCP (P=0.951 and P=0.946, respectively).
IMA is acknowledged as an indirect indicator of increased oxidative stress [13]. Ischemia induces a cascade of inflammatory reactions that lead to ROS generation [12]. Although several studies have associated IMA to various ischemia-related conditions such as acute coronary syndrome, and liver, brain, kidney, and intestinal ischemia in adults, limited data exists on the relationship between IMA and IBD. Kaplan et al. [14], who measured IMA using ELISA, identified that serum IMA levels were significantly higher in patients with IBD than those in the control group. Another study that measured IMA with the albumin cobalt binding method demonstrated that IMA levels were higher in patients with IBD than in healthy controls as well [15]. Additionally, it showed that IMA levels in patients with UC was higher than that in patients with CD; the authors concluded that IMA levels may have increased because of the hypoxia that occurs in tissues after intestinal microvascular ischemia, which is responsible for the classical clinical features of IBD.
Intense bowel inflammation in IBD is accompanied by a demonstrable acute-phase response in the serum. Some inflammation serum markers have been extensively validated in IBD, with CRP and ESR being the most widely employed [4]. FCP is a well-researched calcium-containing protein released into the lumen that is excreted in feces during acute and chronic inflammation and is noninvasive method that presents high sensitivity and specificity for the identification of inflammation in IBD; additionally, FCP shows a very high concordance rate regarding intestinal inflammation compared to endoscopy or biopsy [20,21]. However, in clinical practice, FCP has limitations, such as discomfort in the collection and low patient compliance with the fecal test. In this study, IMA was identified as a significant predictor of endoscopic non-MH. When the representative inflammatory markers were compared between the MH and non-MH groups, significant differences were observed only between IMA and FCP; that is, the IMA and FCP levels increased in the non-MH group. However, no difference in ESR and CRP levels were identified in this study. This is consistent with the results of previous studies; FCP was the most specific indicator of endoscopic activity in IBD. In this study, FCP levels showed statistical significance in predicting endoscopic MH.
Notably, IMA was identified as a new marker that reflects endoscopic activity in the same study sample. However, it should be noted that no significant correlation was found between IMA and FCP; this suggests that these 2 indicators may complement each other in predicting endoscopic MH. Therefore, in this study, IMA and FCP were calculated together to derive a new index, and ROC curve analysis was used to compare endoscopic non-MH in the study subjects. When the Ln(FCP)+ IMA/10 index was calculated, considering the numerical difference between the 2 indices, and applied to the statistics, the AUC of the ROC increased numerically. However, owing to the small sample size, no significant difference was identified compared with the AUC of either FCP or IMA.
This study had several limitations. The first and most important was its cross-sectional design and the small number of patients in both IBD groups, which may have influenced the findings. Second, because the IMA measurement method has not been universally validated, directly comparing the IMA results obtained at our laboratory center with those of previous studies is difficult. Third, as this study was conducted in a cross-sectional setting, it did not reveal changes in IMA during the acute phase of IBD. To act as a clinically significant marker of disease activity, longitudinal studies analyzing the differences in IMA levels are warranted in the future. However, as a new serum marker that reflects the endoscopic healing of IBD, the results showing a predictive value close to that of FCP are significant. Therefore, IMA may be a candidate serum biomarker for predicting endoscopic MH in IBD.
ADDITIONAL INFORMATION
Funding Source
This work was supported by the National Research Foundation (NRF) grant funded by the Korea government (MSIT), awarded to Lee SB (No. 2020R1G1A1006795).
Author Contributions
Conceptualization: Lee SB, Park SH (5th). Data curation: Lee SB, Park SH (5th). Formal analysis: Lee SB, Park SH (5th). Funding acquisition: Lee SB. Investigation: Kim HK, Park SH (3rd), Lim JH. Methodology: Kim HK, Park SH (3rd), Lim JH. Supervision: Park SH (5th). Writing - original draft: Lee SB, Kim HK. Writing - review & editing: Park SH (5th). Approval of final manuscript: all authors.
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Table 1.
Comparison of Ischemia-Modified Albumin Levels Based on Endoscopic Mucosal Healing in Patients with Ulcerative Colitis and Crohnâs Disease
Supplementary Fig. 1.
Levels of ischemia-modified albumin according to endoscopic mucosal healing of ulcerative colitis (A) and Crohnâs disease (B). The P-value was derived using the Mann-Whitney U test.
Supplementary Fig. 2.
Comparison of receiver operating characteristic curve and area under curve (AUC) analyses of ischemia-modified albumin (IMA) and fecal calprotectin (FCP), by various calculation formulas.
Fig. 1.
Levels of ischemia-modified albumin (A) and fecal calprotectin (B) according to endoscopic mucosal healing of inflammatory bowel disease. The P-value was derived using the Mann-Whitney U test.
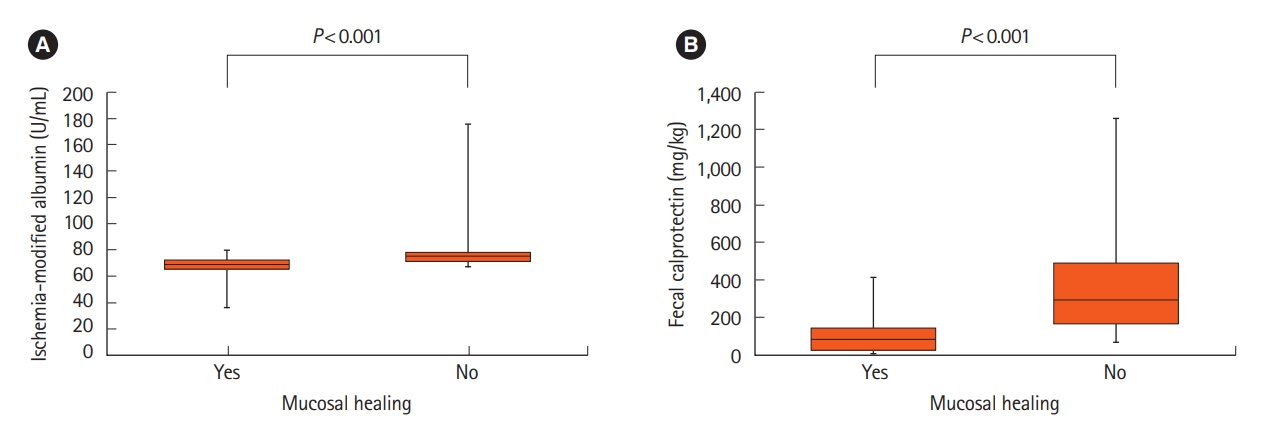
Fig. 3.
Receiver operating characteristic curve and area under curve (AUC) analyses of ischemia-modified albumin (IMA) and fecal calprotectin (FCP). CI, confidence interval.

Table 1.
Baseline Characteristics of the Study Population
| Characteristic | Total patients (n = 48) |
|---|---|
| Age at diagnosis (yr), median (IQR) | 29 (22-45) |
| Sex, No. (%) | |
| âMale | 29 (60.4) |
| âFemale | 19 (39.6) |
| Diagnosis, No. (%) | |
| âUlcerative colitis | 20 (41.7) |
| ââE1/E2/E3a | 0/5/15 |
| âCrohnâs disease | 28 (58.3) |
| ââL1/L2/L3a | 5/0/23 |
| Concomitant medication, No. (%) | |
| â5-Aminosalicyclates | 12 (25.0) |
| âImmunomodulator (AZA/6-MP) | 15 (31.3) |
| âAnti-TNF-Îą antibody | 7 (14.6) |
| âVedolizumab | 9 (18.8) |
| âUstekinumab | 4 (8.3) |
| âTofacitinib | 1 (2.1) |
| Laboratory data, median (IQR) | |
| âIschemia-modified albumin (U/mL) | 72.3 (68.7-77.7) |
| âFecal calprotectin (mg/kg) | 165.5 (87.7-332.1) |
| âESR (mm/hr) | 11 (5-35) |
| âSerum CRP (mg/dL) | 0.18 (0.07-0.59) |
| âSerum albumin (g/dL) | 4.4 (4.1-4.6) |
a Subgroups were divided according to the Montreal classification [19].
Table 2.
Comparison of Baseline Characteristics According to Endoscopic Mucosal Healing
| Characteristic |
Endoscopic mucosal healing |
P-value | ||
|---|---|---|---|---|
| Yes (n=23) | No (n=25) | |||
| Age at diagnosis (yr), median (IQR)a | 30 (25-45) | 28 (20-46) | 0.861 | |
| Sex, No. (%) | 0.951 | |||
| Male | 14 (60.9) | 15 (60.0) | ||
| Female | 9 (39.1) | 10 (40.0) | ||
| Diagnosis, No. (%) | 0.157 | |||
| Ulcerative colitis | 12 (52.2) | 8 (32.0) | ||
| Crohnâs disease | 11 (47.8) | 17 (68.0) | ||
| Concomitant medications, No. (%) | 0.754 | |||
| 5-Aminosalicyclates | 6 (26.1) | 6 (24.0) | ||
| Immunomodulator (AZA/6-MP) | 6 (26.1) | 9 (36.0) | ||
| Biologics and tofacitinib | 11 (47.8) | 10 (40.0) | ||
| Biochemical markers, median (IQR)a | ||||
| Ischemia-modified albumin (U/mL) | 69.3 (65.2-72.7) | 75.5 (70.9-78.2) | < 0.001 | |
| Fecal calprotectin (mg/kg) | 82.4 (24.8-146.3) | 292.6 (165.5-521.4) | < 0.001 | |
| ESR (mm/hr) | 10 (4-30) | 12 (6-35) | 0.516 | |
| Serum CRP (mg/dL) | 0.12 (0.04-0.43) | 0.33 (0.10-0.89) | 0.040 | |
| Serum albumin (g/dL) | 4.4 (4.1-4.8) | 4.4 (4.0-4.5) | 0.225 | |
Table 3.
Association between Biochemical Markers and Endoscopic Mucosal Healing of Inflammatory Bowel Disease
REFERENCES
1. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021;160:1570-1583.


2. Pineton de Chambrun G, Peyrin-Biroulet L, LĂŠmann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol 2010;7:15-29.



3. Sood A, Mahajan R, Singh A, Midha V, Mehta V. Endoscopy for assessment of mucosal healing in ulcerative colitis: time bound or response guided? Intest Res 2022;20:297-302.




4. Nardone OM, Shivaji UN, Ferruzza V, Ghosh S, Iacucci M. Soluble blood markers of mucosal healing in inflammatory bowel disease: the future of noninvasive monitoring. Inflamm Bowel Dis 2020;26:961-969.



5. Moriichi K, Fujiya M, Okumura T. The endoscopic diagnosis of mucosal healing and deep remission in inflammatory bowel disease. Dig Endosc 2021;33:1008-1023.



6. Krzystek-Korpacka M, KempiĹski R, Bromke M, Neubauer K. Biochemical biomarkers of mucosal healing for inflammatory bowel disease in adults. Diagnostics (Basel) 2020;10:367.



7. State M, Negreanu L, Voiosu T, Voiosu A, Balanescu P, Mateescu RB. Surrogate markers of mucosal healing in inflammatory bowel disease: a systematic review. World J Gastroenterol 2021;27:1828-1840.



8. Jeong Y, Jeon SR, Kim HG, et al. The role of platelet to lymphocyte ratio and neutrophil to lymphocyte ratio in ulcerative colitis. Intest Res 2021;19:62-70.




9. Con D, Andrew B, Nicolaides S, van Langenberg DR, Vasudevan A. Biomarker dynamics during infliximab salvage for acute severe ulcerative colitis: C-reactive protein (CRP)-lymphocyte ratio and CRP-albumin ratio are useful in predicting colectomy. Intest Res 2022;20:101-113.




10. Pavlick KP, Laroux FS, Fuseler J, et al. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med 2002;33:311-322.

11. Rezaie A, Parker RD, Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci 2007;52:2015-2021.



12. Bhagavan NV, Lai EM, Rios PA, et al. Evaluation of human serum albumin cobalt binding assay for the assessment of myocardial ischemia and myocardial infarction. Clin Chem 2003;49:581-585.



13. Shevtsova A, Gordiienko I, Tkachenko V, Ushakova G. Ischemia-modified albumin: origins and clinical implications. Dis Markers 2021;2021:9945424.




14. Kaplan M, Yuksel M, Ates I, et al. Is ischemia modified albumin a disease activity marker for inflammatory bowel diseases? J Gastroenterol Hepatol 2016;31:1120-1125.



15. Guntas G, Sahin A, Duran S, et al. Evaluation of ischemia-modified albumin in patients with inflammatory bowel disease. Clin Lab 2017;63:341-347.


16. Omma A, Sandikci SC, Colak S, Tecer D, Yucel C, Ozbalkan Z. Serum calprotectin and ischemia modified albumin levels as markers of disease activity in Behçetâs disease. Postepy Dermatol Alergol 2018;35:609-613.



17. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohnâs disease. N Engl J Med 2004;350:876-885.


18. Daperno M, DâHaens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohnâs disease: the SES-CD. Gastrointest Endosc 2004;60:505-512.


19. Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemiaa preliminary report. J Emerg Med 2000;19:311-315.


20. Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis 2013;19:332-341.











