 |
 |
- Search
| Intest Res > Volume 22(1); 2024 > Article |
|
Abstract
Background/Aims
Methods
Results
ADDITIONAL INFORMATION
Funding Source
The work was supported by the Japan Foundation for Applied Enzymology, JSIBD Grants-in-Aid for IBD Research and JSPS Grant-in-Aid for Scientific Research C (22K08026).
Conflict of Interest
Tsuchiya K has received honoraria (lecture fees) from Takeda Pharmaceutical Co., Ltd. No other potential conflicts of interest relevant to this article were reported.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Author Contributions
Conceptualization: Akiyama S, Tsuchiya K. Data curation; Formal analysis: Akiyama S, Onoda T, Moue S. Data interpretation: Akiyama S, Onoda T, Moue S, Enomoto T, Tsuchiya K. Funding acquisition: Akiyama S, Onoda T, Moue S, Sakamoto N, Sakamoto T, Suzuki H, Enomoto T, Matsubara D, Oda T, Tsuchiya K. Writing - original draft: Akiyama S, Onoda T, Moue S. Writing - review & editing: Akiyama S, Onoda T, Moue S, Enomoto T, Tsuchiya K. Approval of final manuscript: all authors.
Additional Contributions
The authors would like to thank Tsukuba Pathological Analysis Support Service (http://www.hosp.tsukuba.ac.jp/outpatient/facility/kisokenkyu) for the histological staining and Enago (www.enago.jp) for the English language revie
Supplementary Material
Supplementary┬ĀTable┬Ā1.
Supplementary┬ĀTable┬Ā3.
Supplementary┬ĀTable┬Ā5.
Supplementary┬ĀTable┬Ā6.
Supplementary┬ĀTable┬Ā7.
Supplementary┬ĀFig.┬Ā1.
Supplementary┬ĀFig.┬Ā2.
Supplementary┬ĀFig.┬Ā3.
Supplementary┬ĀFig.┬Ā4.
Supplementary┬ĀFig.┬Ā5.
Fig.┬Ā1.
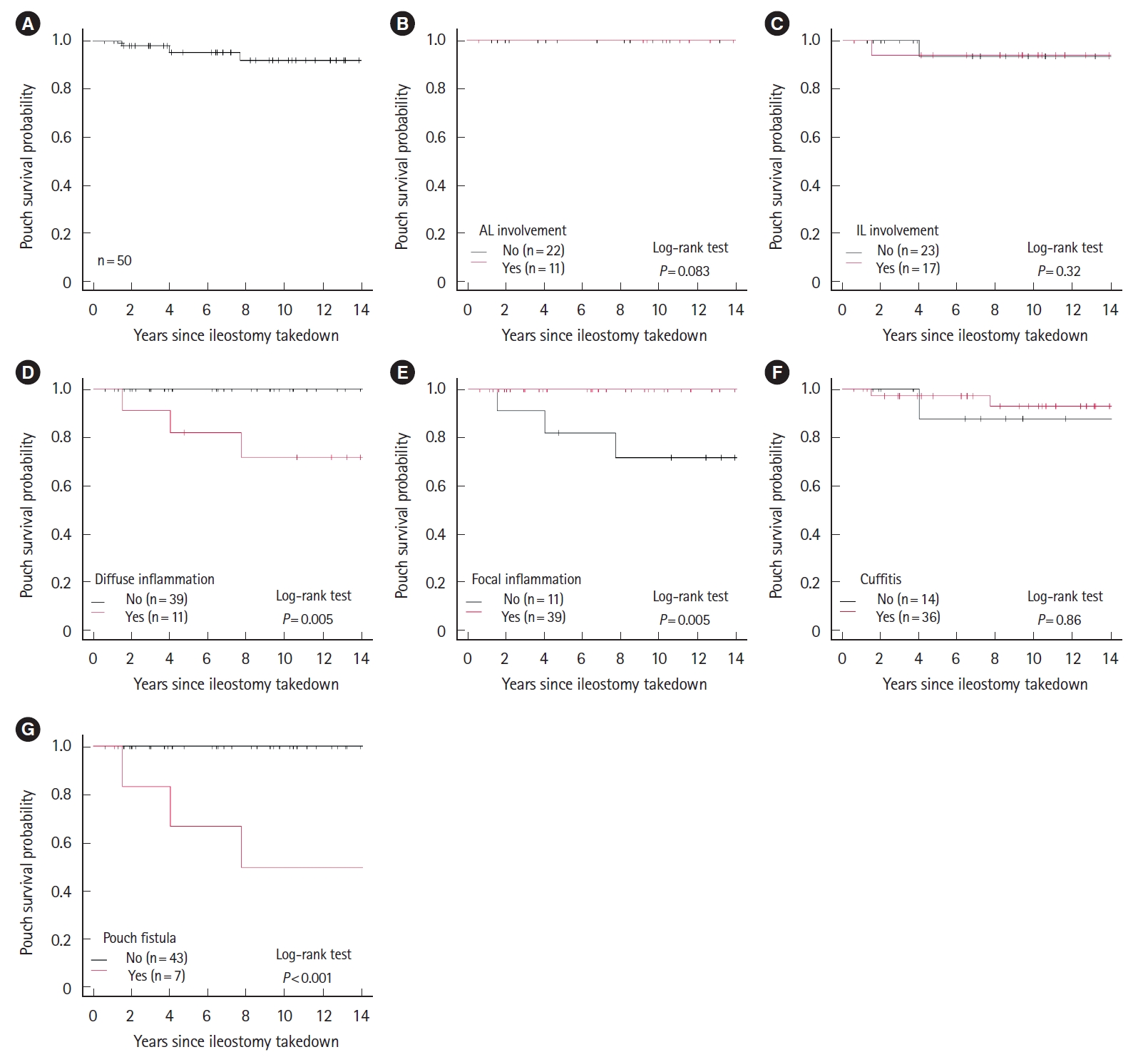
Fig.┬Ā2.

Fig.┬Ā3.

Table┬Ā1.
Table┬Ā2.
Table┬Ā3.
| Variable | Category | Low HID group (n = 10) | High HID group (n = 13) | P-valuea |
|---|---|---|---|---|
| Age at diagnosis (yr) | 29.5 (13-43) | 21.0 (9-47) | 0.054 | |
| Age at colectomy (yr) | 38 (14-46) | 30.0 (16-51) | 0.402 | |
| Sex | Male | 4 (40) | 6 (46.2) | 1.000 |
| Female | 6 (60) | 7 (53.8) | ||
| Body mass index (kg/m2) | 21.7 (19.1-25.8) | 20.3 (17.0-24.2) | 0.063 | |
| Ever-smoker | Yes | 2 (20) | 2 (15.4) | 1.000 |
| No | 8 (80) | 11 (84.6) | ||
| Family history of IBD | Yes | 0 | 2 (15.4) | 0.486 |
| No | 10 (100) | 11 (84.6) | ||
| Disease duration until surgery (yr) | 1.1 (0.08-14.8) | 7.1 (0.25-14.3) | 0.032 | |
| Disease extent | E1 | 0 | 0 | 1.000 |
| E2 | 1 (10) | 1 (7.7) | ||
| E3 | 9 (90) | 12 (92.3) | ||
| Dysplasia/colorectal cancer | Yes | 0 | 2 (15.4) | 0.486 |
| No | 10 (100) | 11 (84.6) | ||
| Stage of IPAA | 1-stage | 0 | 5 (38.5) | 0.014 |
| 2-stage | 10 (100) | 6 (46.2) | ||
| 3-stage | 0 | 2 (15.4) | ||
| Anastomosis type | Staple | 10 (100) | 11 (84.6) | 0.486 |
| Hand-sewn | 0 | 2 (15.4) | ||
| Use of preoperative anti-TNF inhibitors | Yes | 1 (10) | 1 (7.7) | 1.000 |
| No | 9 (90) | 12 (92.3) |
Table┬Ā4.
| Variable | Category | Low HID group (n=10) | High HID group (n=13) | P-valuea |
|---|---|---|---|---|
| Duration between ileostomy takedown and initial endoscopy (mo) | 7.0 (0.50-22.9) | 8.4 (3.1-180) | 0.556 | |
| PDAI score at initial endoscopy | 0.50 (0-2.0) | 1.0 (0-2.0) | 0.426 | |
| No. of endoscopic examinations per patient | 11.5 (9.0-14) | 11 (1.0-18) | 0.574 | |
| Follow-up time (yr) | 12.4 (8.5-15.7) | 12.4 (2.0-16.3) | 0.877 | |
| Postoperative ciprofloxacin | Yes | 1 (10) | 3 (23.1) | 0.604 |
| No | 9 (90) | 10 (76.9) | ||
| Postoperative metronidazole | Yes | 5 (50) | 11 (84.6) | 0.169 |
| No | 5 (50) | 2 (15.4) | ||
| Postoperative oral mesalamine | Yes | 5 (50) | 7 (53.8) | 1.000 |
| No | 5 (50) | 6 (46.2) | ||
| Postoperative mesalamine enema or suppository | Yes | 1 (10) | 6 (46.2) | 0.089 |
| No | 9 (90) | 7 (53.8) | ||
| Postoperative steroid enema or suppository | Yes | 2 (20) | 2 (15.4) | 1.000 |
| No | 8 (80) | 11 (84.6) | ||
| Diverting loop ileostomy | Yes | 1 (10) | 1 (7.7) | 1.000 |
| No | 9 (90) | 12 (92.3) | ||
| Chronic pouchitis | Yes | 5 (50) | 9 (69.2) | 0.417 |
| No | 5 (50) | 4 (30.8) | ||
| Chicago classification | Normal | 0 | 0 | - |
| Afferent limb involvement | 5 (50) | 3 (30.0) | 0.650 | |
| Inlet involvement | 5 (50) | 5 (45.5) | 1.000 | |
| Diffuse inflammation | 2 (20) | 5 (38.5) | 0.405 | |
| Focal inflammation | 8 (80) | 8 (61.5) | 0.405 | |
| Cuffitis | 7 (70) | 10 (76.9) | 1.000 | |
| Fistulas | 1 (10) | 2 (15.4) | 1.000 | |
| Diffuse inflammation and/or fistulas | 2 (20) | 6 (46.2) | 0.379 |








