 |
 |
- Search
| Intest Res > Volume 12(3); 2014 > Article |
|
Abstract
Background/Aims
Infliximab was introduced recently as a rescue therapy for ulcerative colitis (UC) patients refractory to conventional treatments such as therapy with 5-amiono salicylic acids (5-ASA), immune modulators, and corticosteroids. However, there is insufficient data about its efficacy and safety in Korea.
Methods
From 7 tertiary referral hospitals, 33 patients who were treated with infliximab for moderate to severe (Mayo score 6-12) UC refractory to conventional treatment were recruited to this study. Clinical remission was defined as a total Mayo score of 2 or lower and every subscore less than 2. Partial response was defined as a decrease of Mayo score at least 3 points from baseline.
Results
Twenty-three patients (69.7%) showed clinical remission and 29 patients (87.8%) showed partial response in the observation period. When the remission and non-remission groups were compared in univariate analysis, only a higher total Mayo score at base line (11.0±0.9 vs. 9.9±1.5; P=0.04) was related to remission. The remission maintenance rate decreased with time in the Kaplan-Meier analysis. Two patients experienced re-remission after the first remission followed by aggravation during infliximab treatment. Three patients stopped infliximab treatment owing to adverse events including rhabdomyolysis, pneumonia, and fever of unknown origin.
Ulcerative colitis (UC) is a chronic IBD, and its cause is unknown. It is confined to the mucosa and submucosa in the colon and characterized by repeated relapse and remission. UC can deteriorate the quality of life owing to relapsing symptoms including abdominal pain, bloody diarrhea, urgency, etc., and leading to complications such as colon cancer in the long-term.1 Therefore, the management and treatment of this disease are important. Since the first incidence of UC was reported in the 1970s in Korea, its incidence and prevalence have increased continuously.2,3 However, consistent management and examination are essential after diagnosis because, at present, there is no curative treatment for UC. Drugs used to treat UC include 5-aminosalicylic acid (5-ASA), steroids, immunosuppressants (azathioprine [AZP], 6-mercaptopurine [6-MP]), and others drugs. Patients with severe UC can be temporarily managed with corticosteroid injections.4 The association of infections with UC, such as infection with cytomegalovirus, needs to be examined in severe UC patients refractory to corticosteroids;5,6,7 immunosuppressants including cyclosporine, infliximab, and other biological agents can be used concurrently.4 Cyclosporine tends to work more quickly than other immunosuppressive medicines. However, it needs to be used cautiously because of the risk of side effects such as nephrotoxicity, epilepsy, gingival hyperplasia, hypertrichosis, and other opportunistic infections, and it cannot be used in the long-term to maintain remission because of its potential side effects.8,9,10 Infliximab is a monoclonal antibody against tumor necrosis factor alpha (TNF-α). It is known to alleviate histologic inflammation of the mucosa by reducing the secretion of mucosal TNF-α.11,12 Two large-scale randomized controlled trials verified the effectiveness of infliximab in UC.13 However, infliximab is rarely used in patients with severe UC and information on its effects and adverse events are insufficient in Korea.
Therefore, this study aimed to examine the effects and adverse reactions of infliximab and identify predictive factors of response.
This multi-center study retrospectively reviewed the medical records of patients treated with infliximab for UC in the Department of Gastroenterology of 7 tertiary referral hospitals in Korea from January 2007 to December 31, 2011.
Based on the presence of remission at least once during the observation period, the patients were divided into 2 groups. Based on the medical records, patients were examined with gender, age at onset of UC, family history of IBD, initial symptom, extension of lesion, smoking status, opportunistic infections (tuberculosis, human immunodeficiency virus, HBV), white blood cell count, hemoglobin (Hb), platelet, ESR, CRP, and blood test results including albumin level at first use of infliximab. Age at administration of infliximab, drugs used prior to infliximab, indication for infliximab, concurrently administered medicines, number and dose of infliximab, cessation of drug, discontinuation of treatment, remission and partial response, adverse reaction, aggravation and death were examined. Based on the extent of the lesions, UC was classified into proctitis, left-sided colitis, and pancolitis. Medicines administered concurrently or prior to infliximab including 5-ASA, steroid, AZP, 6-MP, methotrexate, antibiotics, and others were noted.
Indications for infliximab treatment were categorized into steroid dependency, steroid refractoriness, refractoriness and side effects of immunosuppressants, and others, with multiple answers allowed. Steroid-dependent UC was confirmed if prednisolone was not tapered to less than 10 mg/day within 3 months of starting steroids without recurrent disease or if relapse occurred within 3 months of stopping prednisolone. Steroid-refractory UC was confirmed if there was no improvement despite administration of prednisolone at a dose of 0.75 mg/kg/day for more than 4 weeks. Immunosuppressant intolerance was confirmed if there was relapse or no improvement despite administration of immunosuppressants (AZP: ~1.5-2.5 mg/kg/day, 6-MP: 0.5-1.0 mg/kg/day) for more than 6 months.
UC disease activity was measured using the Mayo score and checked during each visit for infliximab administration. Since endoscopy examination could not be performed at each visit, partial Mayo scores, i.e., Mayo score without endoscopy subscore, were used. Clinical remission was defined as a total Mayo score of 2 or lower and every subscore less than 2, and partial response was defined as a decrease from baseline by at least 3 points.13 Recurrence or aggravation after remission was defined as a partial Mayo score of 5 or higher, an increase from baseline at least 3 points, or additional medication or surgical procedure owing to the development of new symptoms or signs. This study was performed after gaining institutional review board approval from Kangbuk Samsung Hospital.
To identify factors affecting remission, nominal variables were analyzed with Fisher's exact test, while continuous variables were analyzed with Mann-Whitney U-test. In addition, the nominal variables were subjected to Kaplan-Meier analysis and log-rank (Mantel-Cox) test to examine different durations to remission. The cumulative rate of remission maintenance was analyzed by Kaplan-Meier analysis. Statistical analyses were performed using PASW Statistics 17.0 (SPSS Inc., Chicago, IL, USA).
Thirty-three patients with UC were administered infliximab at a dose of 5 mg/kg at 0, 2, 6, and every 8 weeks. The mean number of times of administration was 4.8 times. Clinical remission, at least once, was achieved in 23 patients (69.7%) during the observation period, induced after an average of 1.4 times of infliximab administration. Remission was not achieved in 10 patients (30.3%), with the mean number of times of infliximab administration being 3.7 times.
Differences between the groups were analyzed according to the presence of remission to identify its association with clinical remission, but no association was shown except for Mayo score. In the univariate analysis, the Mayo score was significantly higher by approximately 1 point, as the remission group scored 11 points and the non-remission group scored 9.9 points (P=0.04). Since there were no other significant factors, a multivariate analysis was not performed. There was no patient with disease extension limited to proctitis. Clinical remission had no association with extent of the lesions, CRP, Hb, immunosuppressant use, indication for infliximab, and concurrent medication (Table 1). Factors related to partial response were analyzed in the same manner, but no significant association was found between them and remission. Kaplan-Meier analysis and log-rank test were conducted by taking varying remission onset and remission maintenance rates into consideration. However, there were no statistically significant factors predicting the presence of remission and maintenance rate.
The number of times infliximab was administered varied from 2 to 9 times. After the first administration, 14 patients (42.4%) achieved remission. Moreover, 6 patients (18.1%), 2 patients (6.0%), and 1 patient (3.0%) reached remission at second, third and fourth administrations, respectively. A total of 23 patients (69.7%) achieved remission after an average 1.4 times of administration. Meanwhile, 29 patients (87.8%) showed clinical improvement, including 6 patients (18.1%) with partial response. No patient experienced aggravated symptoms after partial response. Recurrent or aggravated symptoms were observed in 7 remission patients. The partial Mayo scores ranged between 3 and 7 points after aggravation, and no patient underwent surgery. The same unchanged dose of infliximab was consistently administered to 2 patients, as planned, and they achieved re-remission (Fig. 1). Three patients reached remission before the 8th week of treatment, but the remission was followed by relapse. When they were considered not having achieved remission, the partial response and remission rates were 60.6% and 39.4%, respectively on the 8th week according to the intention to treat analysis. When those patients were considered having achieved remission, the partial response and remission rates were 66.6% and 48.4%, respectively.
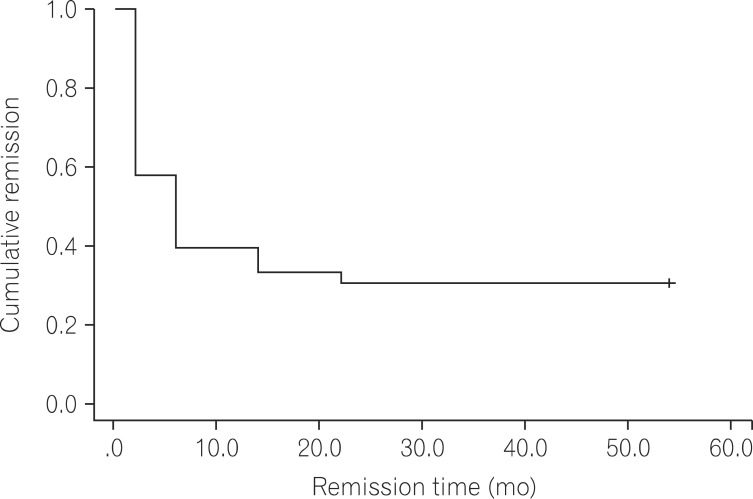
Kaplan-Meier analysis was performed to assess the cumulative rate of remission maintenance. Cumulative remission maintenance rate decreased with time (Fig. 2). No statistical difference was found in the cumulative remission maintenance rate according to initial symptoms, gender, extension, concurrently used medication, smoking status, and indications for infliximab.
Of all the 33 patients, adverse reactions were confirmed in 4 patients (12.1%), as shown in Table 2. None of the patients died during the observation period. Of these, 1 patient developed pancreatitis, while concurrently using AZP and infliximab, so AZP was replaced by 6-MP and concurrently administered with infliximab.
In this study, the use of infliximab clinically improved symptoms in almost 90% of moderate to severe UC patients refractory to steroids or immune modulators, showing a decrease in the baseline partial Mayo score by ≥3 points. Remission was reached in about 70% of all patients once or more than once. In addition, there was no death or surgery during the follow-up. Our results were higher than the response and remission induction rates reported in other studies performed in Korea.14 We assumed this was caused because remission was taken as standard criteria at any time point during the observation period, unlike other previous studies that determined clinical scores only at 8 weeks after the first infliximab administration. Moreover, this study aimed to identify predictive factors associated with the achievement of remission after infliximab administration. However, there was no clinical difference except for high Mayo scores indicating high disease activity at the first administration. Since these results were obtained from univariate analysis only, they were insufficient to be considered significant. Taking into consideration the fact that the CRP level was elevated and albumin level was low at the first administration in the remission group, high disease activity was predicted to have positive effect on remission induction caused by infliximab use. Likewise, a previous study that retrospectively reviewed 90 UC patients suggested that severe UC was a predictive factor for positive response to infliximab.15 However, high disease activity was considered a predictive factor for poor response to infliximab in several previous studies.16,17,18 In contrast, a recent study involving 89 Korean UC patients proposed that a Mayo score of greater than 11 was a predictive factor for nonresponse to infliximab.18 Another recent study involving 134 Korean UC patients reported that CRP levels above 3.0 mg/dL and Hb levels above 11.5 g/dL were positive predictive factors for remission or partial response, and inconsistent results were shown for several factors representing disease activity in predicting the effects of infliximab.14 Therefore, additional studies are crucial to investigate causes for aggravation after remission from various perspectives of immunology, biochemistry, pharmacology, and environmental factors affecting disease progression.
Unlike the previous studies, this study analyzed "remission" only as the standard criteria instead of the remission state 8 weeks after infliximab use. Although remission is achieved before 8 weeks, relapse can occur after 8 weeks. Since the remission rate varies depending on total number and time of administration, as demonstrated by the results of the Kaplan-Meier analysis in this study and those of other large-scale studies, determining the effects and predictive factors of infliximab with "remission" achievement is thought to be more suitable rather than doing so with remission state at certain point of time. It is beneficial to compare remission state at specific point only if the aim of the study is to compare the remission rate between 2 groups, but the purpose of this study was to identify remission predictive factors. In the study, 3 patients reached remission and a short remission was followed by a relapse after the third administration (6 weeks after first administration). The partial Mayo scores for the patients were 5, 7, and 4 points, respectively. Those 3 patients accounted for 9.0% (3/33) of all subjects, and 13.0% (3/23) of all patients who achieved remission. According to the analysis based on 8 weeks of administration, an error can occur in the analysis of response-predictive factors by placing those 3 patients in the non-remission group. This is considered one of the reasons for our results not being comparable to those of previous studies on remission-predictive factors. Considering the fact that repeated remission and relapses can occur, whether remission at a specific point of time can be taken as the standard for identifying remission predictive factors instead of "remission" achievement needs to be carefully determined. To the best of the authors' knowledge, this issue has not been addressed in previous studies on the efficacy and response-predictive factors of infliximab in patients with UC, probably because remission achievement at a certain point of time was overlooked as massive data sets were analyzed in those studies. Although this study had a small sample size, new findings were revealed through in-depth analyses.
According to the Kaplan-Meier graph showing the remission rate of infliximab, the cumulative rate of remission maintenance decreased with time. However, factors related to remission maintenance rate were not identified. Many studies verified clinical improvement by administering infliximab to UC patients refractory to conventional therapies. The clinical improvement showed a decreasing tendency in both response and cumulative remission maintenance rates with time.13,19,20 Some studies suggested that the decrease in the cumulative rates of remission maintenance was caused by a reduction in the plasma concentrations of infliximab, with the formation of antibodies against infliximab.21 In contrast, antibodies against infliximab present before drug administration were found to be not related to response to infliximab in UC patients.22 Furthermore, large-scale prospective study, a high response rate was observed at weeks 30 and 54 when antibodies were present in the bodies of UC patients.13 Therefore, additional studies are essential to further determine the antibodies and potential factors that cause non-response to infliximab. In this study, severe adverse events to the degree of following discontinuation of treatment occurred in 4 patients, but none of the patients died. Of these patients with side effects, in the case of 1 patient with pancreatitis, AZP was replaced with 6-MP, despite sustained use of infliximab. The adverse event was more likely to be caused by AZP. Therefore, relatively severe adverse reaction occurred in 3 (9.0%) out of 33 patients.
There are some limitations to this study. This study had a small sample size and relatively short follow-up period. Current health insurance coverage of infliximab for UC management is limited for those refractory or intolerant to conventional medications including steroid, AZP, or 6-MP and those with moderate to severe UC with a Mayo score of 6-12 points. Insurance coverage is discontinued for patients with no response to a maximum of 3 doses of infliximab. Therefore, most patients stop taking infliximab after 3 doses, if they are refractory to infliximab. Since response to the drug was mainly observed in the early period of administration in most previous studies, the small sample size is considered as a bigger barrier than the short follow-up period. However, the small sample size enabled in-depth analysis and helped reveal new findings. Moreover, there could have been differences between hospitals and physicians in treating disease owing to the nature of this multi-center study, so efforts were made to resolve this shortcoming by creating the objectified form of data input, considering the current condition of the actual clinical field at the data collection phase.
To sum up, this study analyzed a total of 33 Korean UC patients administered infliximab, and almost 90% showed clinical improvement. Some patients had a relapse followed by remission with time, and adverse events occurred in the case of some patients. It was found that the remitting and relapsing course can be repeated. In Korea, for moderate to severe UC patients refractory to conventional treatment with surgery as the last treatment option, infliximab is an effective and relatively safe treatment option. Further studies are required because the response to infliximab, potential side effects, and remitting and relapsing courses have not yet been fully clarified.
References
1. Staehelin A. Prognosis of ulcerative colitis. Follow-up control of cases from 1942 to 1969. Schweiz Med Wochenschr 1970;100:580-582.PMID: 5420854.

2. Kim SJ, Cho YK, Rhee JE, Yoon CM. Two cases of ulcerative colitis. Korean J Gastroenterol 1970;2:29-33.
3. Yang SK, Hong WS, Min YI, et al. Incidence and prevalence of ulcerative colitis in the Songpa-Kangdong District, Seoul, Korea, 1986-1997. J Gastroenterol Hepatol 2000;15:1037-1042.PMID: 11059934.


4. Choi CH, Kim YH, Kim YS, et al. Guidelines for the management of ulcerative colitis. Korean J Gastroenterol 2012;59:118-140.PMID: 22387836.


5. Wada Y, Matsui T, Matake H, et al. Intractable ulcerative colitis caused by cytomegalovirus infection: a prospective study on prevalence, diagnosis, and treatment. Dis Colon Rectum 2003;46:S59-S65.PMID: 14530660.


6. Criscuoli V, Casa A, Orlando A, et al. Severe acute colitis associated with CMV: a prevalence study. Dig Liver Dis 2004;36:818-820.PMID: 15646428.


7. Kim YS, Kim YH, Kim JS, et al. The prevalence and efficacy of ganciclovir on steroid-refractory ulcerative colitis with cytomegalovirus infection: a prospective multicenter study. J Clin Gastroenterol 2012;46:51-56.PMID: 21552140.


8. Van Assche G, D'Haens G, Noman M, et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology 2003;125:1025-1031.PMID: 14517785.


9. Campbell S, Travis S, Jewell D. Ciclosporin use in acute ulcerative colitis: a long-term experience. Eur J Gastroenterol Hepatol 2005;17:79-84.PMID: 15647646.


10. Haslam N, Hearing SD, Probert CS. Audit of cyclosporin use in inflammatory bowel disease: limited benefits, numerous side-effects. Eur J Gastroenterol Hepatol 2000;12:657-660.PMID: 10912486.


11. Knight DM, Trinh H, Le J, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol 1993;30:1443-1453.PMID: 8232330.


12. Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut 1993;34:1705-1709.PMID: 8031350.



13. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462-2476.PMID: 16339095.


14. Lee KM, Jeen YT, Cho JY, et al. Efficacy, safety, and predictors of response to infliximab therapy for ulcerative colitis: a Korean multicenter retrospective study. J Gastroenterol Hepatol 2013;28:1829-1833.PMID: 23829336.


15. Jurgens M, Laubender RP, Hartl F, et al. Disease activity, ANCA, and IL23R genotype status determine early response to infliximab in patients with ulcerative colitis. Am J Gastroenterol 2010;105:1811-1819.PMID: 20197757.



16. Oussalah A, Evesque L, Laharie D, et al. A multicenter experience with infliximab for ulcerative colitis: outcomes and predictors of response, optimization, colectomy, and hospitalization. Am J Gastroenterol 2010;105:2617-2625.PMID: 20736936.



17. Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut 2010;59:49-54.PMID: 19651627.


18. Park SH, Yang SK, Hong SM, et al. Severe disease activity and cytomegalovirus colitis are predictive of a nonresponse to infliximab in patients with ulcerative colitis. Dig Dis Sci 2013;58:3592-3599.PMID: 23979435.


19. Chaparro M, Burgueno P, Iglesias E, et al. Infliximab salvage therapy after failure of ciclosporin in corticosteroid-refractory ulcerative colitis: a multicentre study. Aliment Pharmacol Ther 2012;35:275-283.PMID: 22142227.


20. Sjoberg M, Walch A, Meshkat M, et al. Infliximab or cyclosporine as rescue therapy in hospitalized patients with steroid-refractory ulcerative colitis: a retrospective observational study. Inflamm Bowel Dis 2012;18:212-218.PMID: 21438096.


21. Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol 2013;108:40-47.PMID: 23147525.



22. Steenholdt C, Palarasah Y, Bendtzen K, et al. Pre-existing IgG antibodies cross-reacting with the Fab region of infliximab predict efficacy and safety of infliximab therapy in inflammatory bowel disease. Aliment Pharmacol Ther 2013;37:1172-1183.PMID: 23650912.


Fig. 1
A schematic clinical course of patients. Schematic overview of the clinical course of UC in the study patients during the observation period, regardless of the number of infliximab injections. The dichotomous status of UC is shown as in remission (+) or not (-).
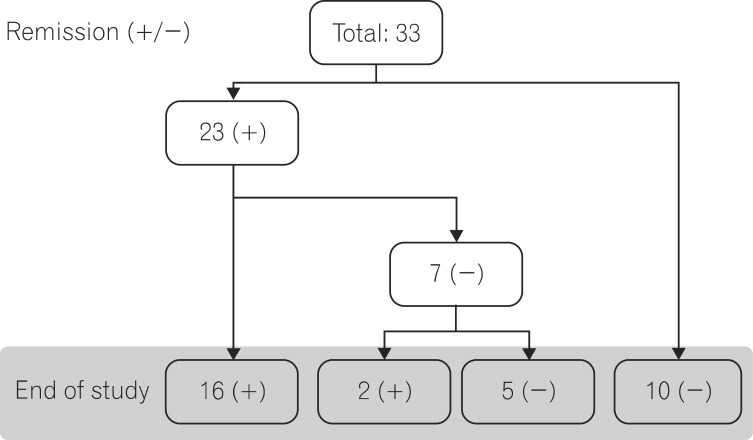
Fig. 2
Kaplan-Meier graph of cumulative remission. The graph shows progressive decrease in cumulative remission rate with time during infliximab treatment.
- TOOLS








