INTRODUCTION
Ulcerative colitis (UC) is a chronic relapsing disease characterized by mucosal inflammation of the colon [
1,
2]. Typical symptoms include rectal bleeding, diarrhea, and bowel urgency which negatively impact patients’ quality of life [
3]. UC is classed as a global disease, and although the incidence is stabilizing in Western countries [
4], the prevlence of UC has risen substantially in Japan over the previous 2 decades [
5].
The goal of treatment for UC is to induce and maintain remission. Despite significant treatment advances, current therapies pose limitations and/or lose clinical efficacy over time [
6]. Hence, a need remains for new therapeutics with an adequate benefit: risk profile to manage this complex disease.
The interleukin (IL)-23 axis plays a significant role in the pathogenesis of UC [
7] and has thus become an important target for drug development. IL-23 contains a unique p19 subunit and a common p40 subunit which it shares with IL-12 [
8]. Mirikizumab is a humanized immunoglobulin G4-variant monoclonal antibody that specifically binds to the p19 subunit of IL-23. In phase 2 trials, mirikizumab demonstrated efficacy in patients with moderately to severely active UC, including in Japanese patients [
9,
10].
We recently reported the findings from phase 3, randomized, global, double-blind, placebo-controlled studies for induction (LUCENT-1, NCT03518086) and maintenance (LUCENT-2, NCT03524092) treatment with mirikizumab in UC [
11]. These were the first reported phase 3 studies describing the safety and efficacy of targeting the p19 subunit of IL-23 for the treatment of UC. LUCENT-1 and LUCENT-2 demonstrated mirikizumab was more effective compared with placebo in inducing and maintaining clinical remission in patients with moderately to severely active UC, and were the first phase 3 studies in UC to evaluate bowel urgency and demonstrate the efficacy of mirikizumab in improving bowel urgency severity and bowel urgency numeric rating scale (NRS) of 0 or 1 [
11]. The frequency of treatment-emergent adverse events (TEAEs) was similar across mirikizumab and placebo groups, with no new major safety concerns identified [
11]. In the current study, we describe efficacy and safety of mirikizumab compared with placebo in Japanese patients with moderately to severely active UC from the phase 3 LUCENT-1 and LUCENT-2 trials.
METHODS
1. Study Design
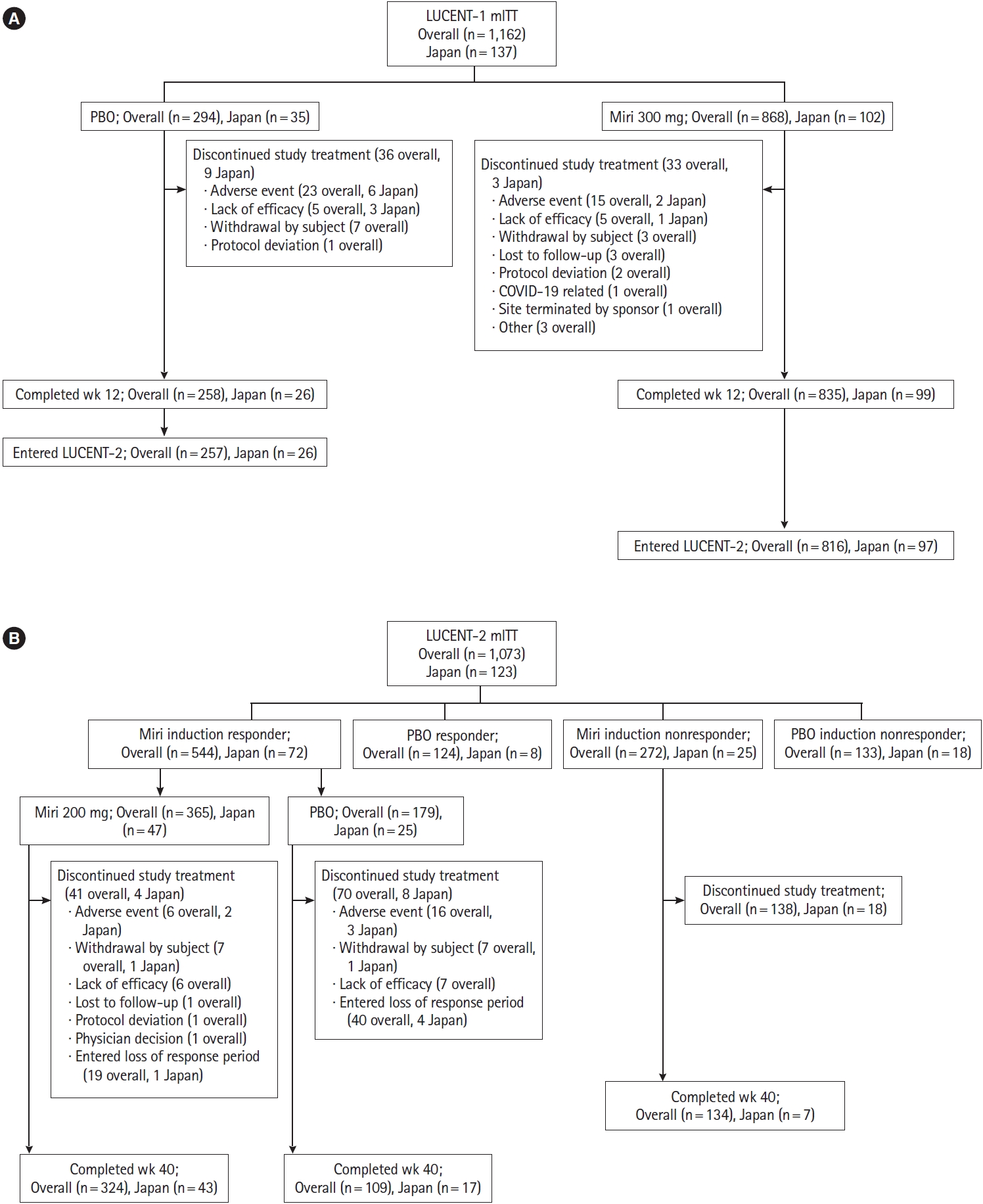
LUCENT-1 and LUCENT-2 were both phase 3, multicenter, double-blind, randomized, parallel-arm, placebo-controlled trials. LUCENT-1 was a 12-week induction trial and LUCENT-2 was a 40-week maintenance trial, comprising a total of 52 weeks of treatment. The study design has been previously described [
9] and is shown in
Supplementary Fig. 1. Study design modifications specific to Japanese patients are outlined in the
Supplementary Material.
Briefly, in LUCENT-1, eligible patients were randomized 3:1 to receive mirikizumab 300 mg or placebo via intravenous (IV) infusion every 4 weeks (Q4W) at weeks 0, 4, and 8.
Randomization was stratified by biologic or tofacitinib-failure status (yes/no), baseline corticosteroid use (yes/no), baseline disease activity (modified Mayo score [4-6] or [7-9]), and region (North America/Europe/other). Assignment to treatment groups was determined by a computer-generated random sequence using an interactive web-response system. Anti-tumor necrosis factor antibodies and Janus kinase inhibitors were required to be discontinued at least 8 weeks and at least 4 weeks prior to the screening endoscopy, respectively, and were subsequently prohibited throughout the duration of the study. As per the study design, only patients who achieved a clinical response to mirikizumab treatment at the end of the induction study (LUCENT-1) were randomized 2:1 to doubleblind treatment with either mirikizumab 200 mg subcutaneously (SC) Q4W or placebo SC Q4W during the maintenance study (LUCENT-2). Patients who did not achieve a clinical response at the end of the induction study were not included in the double-blind treatment arms during the maintenance study.
Randomization in LUCENT-2 was stratified by biologic or tofacitinib-failure status (yes/no), induction remission status (yes/no), corticosteroid use (yes/no), and region (North America/Europe/other). Patients who achieved a clinical response to placebo at the end of the induction study continued blinded placebo during the maintenance study. Induction clinical responders who experienced a loss of response to either mirikizumab or placebo at or after week 12 of the maintenance study could receive rescue induction therapy with open-label mirikizumab 300 mg IV Q4W for 3 doses. Patients with loss of response who, in the opinion of the investigator, had a clinical benefit from rescue therapy, were considered for transition to the long-term extension study. Patients without a clinical response to mirikizumab or placebo in the induction study were enrolled in the maintenance study as nonresponders, receiving open-label extended induction dosing with mirikizumab 300 mg IV Q4W for 3 doses. Those who achieved clinical response following extended induction dosing at week 12 of the maintenance study received open-label mirikizumab 200 mg SC Q4W maintenance dosing through week 40 while nonresponders discontinued the study.
The study was conducted in accordance with the International Conference on Harmonisation for Good Clinical Practice and the Declaration of Helsinki. The study protocols were approved by the institutional review board responsible for oversight at each center. All patients provided written informed consent to participate in the study.
2. Participants
Adults (aged 18-80 years) with moderately to severely active UC at screening (modified Mayo score of 4-9 [range 0 to 9] with an endoscopic subscore of 2-3 [range 0 to 3]) were eligible for inclusion in the LUCENT-1 induction study. Eligible patients had an established diagnosis of UC at least 3 months prior to baseline, with endoscopic evidence and histopathology to support the diagnosis, and UC extending beyond the rectum. Patients had inadequate response, loss of response, or intolerance to corticosteroids or immunomodulators (see
Supplementary Material) or had failed or demonstrated an intolerance to a biologic medication or Janus kinase inhibitors. Patients were allowed to use oral 5-aminosalicyclic acid, immunomodulators azathioprine or 6-mercaptopurine, or methotrexate with stable doses required. Patients taking corticosteroids at baseline were required to maintain a stable dose during the induction trial but were required to begin corticosteroid taper upon entering the LUCENT-2 maintenance study as induction responders.
Patients were ineligible for study inclusion if UC was limited to the rectum, if they were diagnosed with other forms of inflammatory bowel disease or an immunodeficiency syndrome which may cause UC-like colonic inflammation, had extensive colonic resection, stricture/stenosis within the small bowel or colon, toxic megacolon, colonic adenoma that had not been removed, dysplasia of colonic mucosa, gastrointestinal cancer, or failed 3 or more biologic therapies (excluding tofacitinib) for UC. Further details of patient inclusion and exclusion criteria for LUCENT-1 and LUCENT-2 have been previously described, and criteria specific to Japanese patients are outlined in the
Supplementary Appendix. Patients who completed the 12-week treatment period (LUCENT-1), irrespective of clinical response status, were eligible to participate in the 40-week maintenance study (LUCENT-2).
3. Efficacy and Safety Evaluation
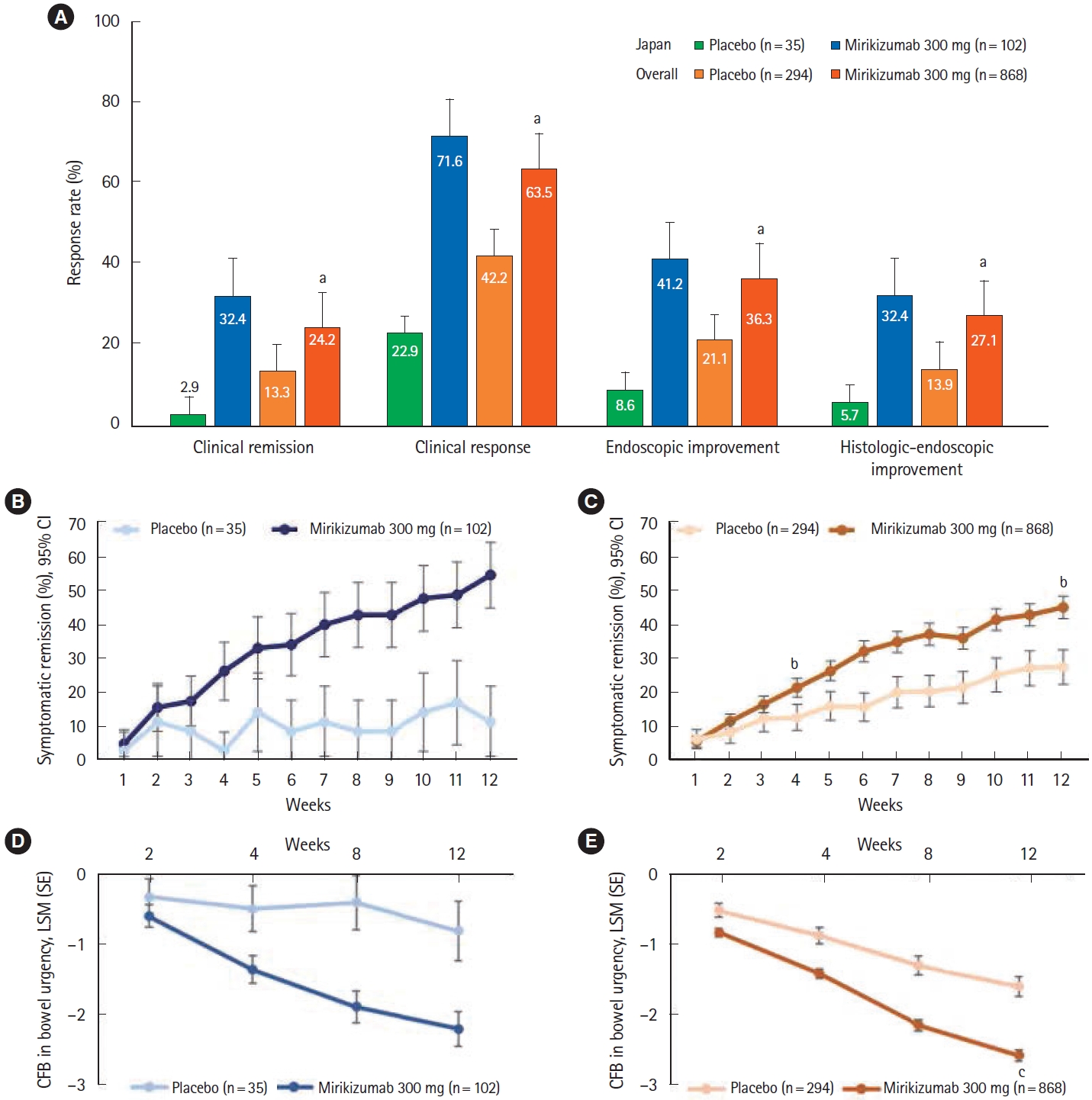
For LUCENT-1, the primary endpoint was the proportion of patients in clinical remission at week 12. Clinical remission was based on the modified Mayo score and defined as: stool frequency subscore =0 (range 0 to 3), or stool frequency =1 with a ≥ 1-point decrease from baseline, rectal bleeding subscore =0 (range 0 to 3), and endoscopic subscore =0 or 1 (excluding friability). Key secondary endpoints at week 12 included alternative clinical remission, clinical response, endoscopic improvement, symptomatic remission (week 4 and week 12), clinical response in the biologic-failed population, histologic-endoscopic mucosal improvement (as demonstrated in colonic biopsy samples), and change from baseline in bowel urgency NRS.
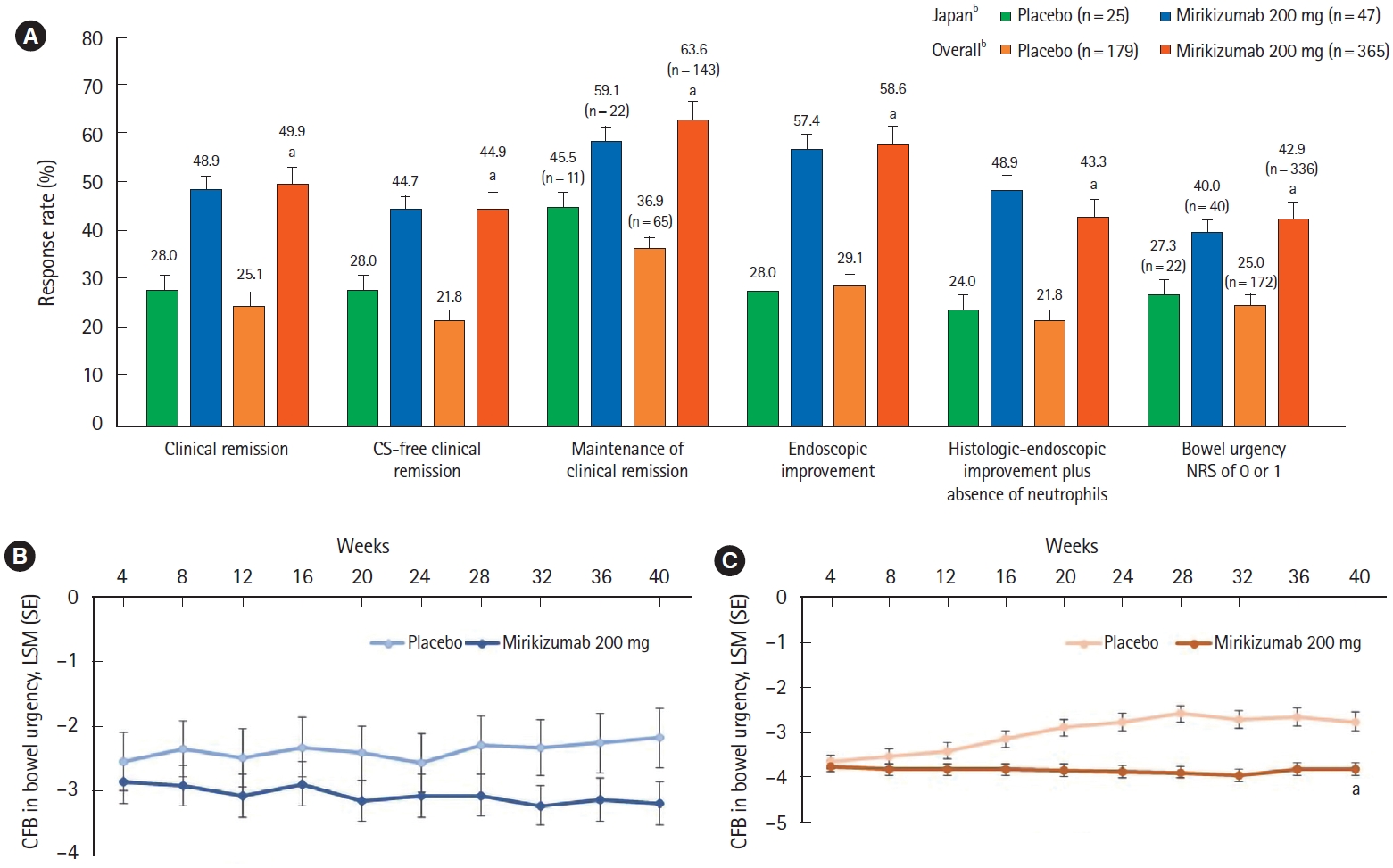
For LUCENT-2, the primary endpoint was the proportion of patients in clinical remission at week 40 from patients who achieved a clinical response with mirikizumab in LUCENT-1. Key secondary endpoints at week 40 included alternative clinical remission among patients induced into clinical response in LUCENT-1, endoscopic improvement, maintenance of clinical remission, corticosteroid-free clinical remission, histologic-endoscopic mucosal improvement plus absence of neutrophils (as demonstrated in colonic biopsy samples), improvement in bowel urgency severity, and bowel urgency NRS of 0 or 1. Bowel urgency NRS of 0 or 1 indicates normal or near normalization of bowel urgency status.
An additional endpoint for LUCENT-1 and LUCENT-2 was the average serum concentration of mirikizumab (full details are outlined in
Supplementary Table 1). A complete list of primary and secondary endpoints and their definitions for both LUCENT-1 and LUCENT-2 are provided in
Supplementary Table 2.
Safety outcomes included TEAEs, study discontinuation due to adverse events (AEs), deaths, serious AEs, AEs of special interest, laboratory values, and vital signs. AEs of special interest included infections (opportunistic and serious), hepatic safety, hypersensitivity, infusion reactions, injection site reactions, cerebrocardiovascular events, malignancies, suicidal ideation, and depression.
4. Statistical Analyses
Efficacy and safety analyses were performed on the modified intent-to-treat populations of LUCENT-1 and LUCENT-2. These populations included all randomized patients who received at least 1 dose of study drug during the respective study. The results of the Japanese subpopulation analyses were summarized descriptively. Consistency in efficacy and safety outcomes between the Japanese subpopulation and overall population were evaluated.
For assessments of the primary endpoints and other cate gorical efficacy endpoints, the Cochran-Mantel-Haenszel test was used to compare the treatment groups while adjusting for stratification factors. For analysis of categorical efficacy and health outcomes variables, patients were considered nonresponders if they did not meet the categorical efficacy criteria, had missing clinical efficacy data at a time point of interest, or took rescue medication prior to the time point of interest. Treatment comparisons of continuous efficacy variables were made using the mixed-effects model for repeated measures analyses, with censoring of data following rescue therapy. Primary and major secondary endpoints for the overall population were tested after adjusting for multiplicity using a predefined graphical procedure with an overall family-wise error rate of 0.00125 for LUCENT-1 and 0.05 for LUCENT-2. For both studies, analyses of hypotheses without multiplicity control, including all analyses in the Japanese subpopulation, are reported with point estimates and 95% confidence intervals, without P-values; the widths of the confidence intervals are not adjusted for multiple testing and should not be used to infer definitive treatment effects.
DISCUSSION
This subgroup analysis of LUCENT-1 and LUCENT-2 demonstrated the efficacy and safety of mirikizumab induction and maintenance therapy in Japanese patients with moderately to severely active UC. The proportions of Japanese patients treated with mirikizumab who achieved clinical remission, clinical response, and endoscopic and histologic-endoscopic improvement during the 12-week induction trial were numerically higher than those who received placebo. Consistent with the overall population, early improvements in symptomatic remission in patients treated with mirikizumab compared with placebo were demonstrated.
Mirikizumab’s efficacy in reducing bowel urgency is particularly encouraging given a recent noninterventional study reported that UC had a significant impact on daily life in 43.5% of Japanese patients who experienced bowel urgency [
12]. Overall, the proportion of participants who stated that UC “significantly impacted” their daily life was highest in participants who experienced bowel urgency or bowel incontinence [
12]. Hence improvements in bowel urgency and bowel incontinence are likely to provide significant benefits in quality of life to a significant proportion of Japanese patients with UC.
Patients who achieved a clinical response to mirikizumab treatment at the end of the induction study could receive double-blind treatment with either mirikizumab 200 mg or placebo during the maintenance study. In the 40-week maintenance trial (total of 52 weeks of treatment), mirikizumab treatment demonstrated maintenance of efficacy as higher proportions of patients treated with mirikizumab achieved clinical remission, corticosteroid-free clinical remission, maintenance of clinical remission, endoscopic and histologic-endoscopic mucosal improvement plus absence of neutrophils, and bowel urgency (NRS of 0 or 1, which indicates normal or near normalization of bowel urgency) compared to placebo. Numerical improvements from baseline in bowel urgency with mirikizumab treatment were observed in the induction phase and maintained through the 40-week maintenance period in comparison to placebo. These findings in Japanese patients with moderately to severely active UC are encouraging. While clinical response and clinical remission are immediate and intermediate treatment targets for UC, respectively, endoscopic healing is a long-term treatment goal as recommended in the Selecting Therapeutic Targets in Inflammatory Bowel Disease II guidelines [
13]. Although patients in the overall population who did not achieve a response to induction dosing (or lost response during maintenance) had numerically lower mirikizumab exposures than patients who achieved response (or did not lose response), due to confounding, it cannot be concluded that these lower exposures caused reduced efficacy or loss of response.
Of note, the histologic improvement component of the maintenance histologic-endoscopic mucosal improvement endpoint required the absence of mucosal neutrophils. This endpoint has been associated with improved long-term outcomes such as reduced incidence of clinical relapse and hospitalization, colectomy, and glucocorticoid use [
14,
15]. Following the maintenance study, approximately 49% of Japanese patients and 43% of the overall population treated with mirikizumab had absence of mucosal neutrophils in colonic biopsies.
Despite the relatively small number of patients in the Japanese subpopulation, the primary and major secondary efficacy outcomes in both the induction and maintenance studies were consistent with the findings in the overall study population [
11]. This consistency is important considering the differences in the genetic background of these populations in terms of the prevalence of susceptibility genes [
16]. In the global LUCENT-1 and LUCENT-2 studies, a significantly greater proportion of patients treated with mirikizumab achieved clinical remission during induction (
P=0.00006) and maintenance (
P<0.001) [
11]. Furthermore, in both the global LUCENT-1 and LUCENT-2 studies, mirikizumab was superior to placebo in all major secondary endpoints including clinical response, endoscopic improvement, histologic-endoscopic improvement, and corticosteroid-free clinical remission (all
P<0.0001), as well as significant improvements in symptomatic remission and bowel urgency [
11]. In the induction phase, the response in the placebo group was lower in the Japanese subpopulation than the overall population. The reasons for this are unclear but may be due to a higher proportion of Japanese patients in the placebo group with a Mayo endoscopic subscore of 3 (severe disease) or longer disease duration.
Mirikizumab treatment during induction and maintenance was well tolerated in Japanese patients. The frequency of TEAEs was similar across mirikizumab and placebo groups in both the induction and maintenance studies. The incidence of serious AEs and study-treatment discontinuations due to AEs was low, and numerically higher in the placebo group compared with the mirikizumab group. The frequency of infections was similar between the mirikizumab and placebo treatment arms. The incidence of serious infections was very low in the induction study and no serious infections were reported in the maintenance study. The safety profile of mirikizumab observed in the Japanese subpopulation was broadly consistent with that observed in the overall population [
11], and in prior studies of mirikizumab in UC [
9,
10], Crohn’s disease [
17], and psoriasis [
18]. In the maintenance study, there was a higher frequency of TEAEs (including AEs of special interest) in the Japanese subpopulation than the overall population in both the mirikizumab and placebo groups. This may be due in part to the higher incidence of nasopharyngitis in the Japanese population. The frequency of malignancies in the Japanese subpopulation was comparable to the overall population. Overall, no new safety concerns were identified in Japanese patients.
The main limitation of this subgroup analysis is the relatively small number of Japanese patients. Furthermore, the 12-week induction and 40-week maintenance periods may limit the detection and interpretation of AEs with low incidence (such as malignancy). The data from long-term extension study (LUCENT-3, NCT03519945) will provide further clarification regarding the long-term efficacy and safety profile of mirikizumab in Japanese patients with UC.
In conclusion, mirikizumab provided clinical benefits and was well tolerated in Japanese patients with moderately to severely active UC. Our findings from this subpopulation analysis of Japanese patients are in alignment with the efficacy and safety findings observed in the overall population. These findings, together with those of the overall population, support the potential of mirikizumab as a promising treatment option in Japanese patients with moderately to severely active UC where, despite advances in available treatments, a substantial unmet need persists.