 |
 |
- Search
| Intest Res > Volume 17(3); 2019 > Article |
|
Abstract
Background/Aims
Methods
Results
NOTES
FINANCIAL SUPPORT
This work was supported by AstraZeneca K.K. and Amgen Inc. Marion Barnett of Edanz Medical Writing provided medical writing assistance, which was funded by AstraZeneca through EMC K.K. in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
CONFLICT OF INTEREST
T.H. has received consultancy fees from AbbVie Japan, JIMRO, Takeda Pharmaceuticals, Mitsubishi Tanabe Pharma, EA Pharma, Eli Lilly Japan KK, Pfizer Japan Inc., Nippon Kayaku Co. Ltd., and Nichi-Iko Pharmaceutical Co. Ltd., and grants from Zeria Pharmaceuticals, Otsuka Holdings Co. Ltd., AbbVie Japan, EA Pharma, and JIMRO. R.G. is an employee of MedImmune, LLC, a subsidiary of AstraZeneca. M.N. is an employee of AstraZeneca K.K. B.S. is a former employee and current shareholder of Amgen Inc. S.N. has received grants from Kyorin Pharmaceutical, Mitsubishi Tanabe Pharma, EA Pharma, AbbVie Japan, Asahi Kasei Medical, JIMRO, Otuka Pharmaceutical Factory, ZERIA Pharmaceuticals, Mochida Pharmaceutical, and Astellas Pharma, and lecture fees from AbbVie Japan, Janssen Pharmaceutical, EA Pharma, Mitsubishi Tanabe Pharma, ZERIA Pharmaceuticals, and Mochida Pharmaceutical. A.M., S.M., S.S., T.A., Y. Sameshima, and Y. Suzuki have no conflicts of interest to declare.
AUTHOR CONTRIBUTION
Conceptualization: Hibi T. Methodology: Hibi T, Gasser RA, Sullivan BA. Formal analysis: Hibi T, Gasser RA, Nii M, Sullivan BA. Investigation: Motoya S, Ashida T, Sai S, Sameshima Y, Nakamura S, Maemoto A, Suzuki Y, Sullivan BA. Writing, review, and editing: all authors. Approval of final manuscript: all authors.
ACKNOWLEDGEMENTS
Fig.┬Ā1.
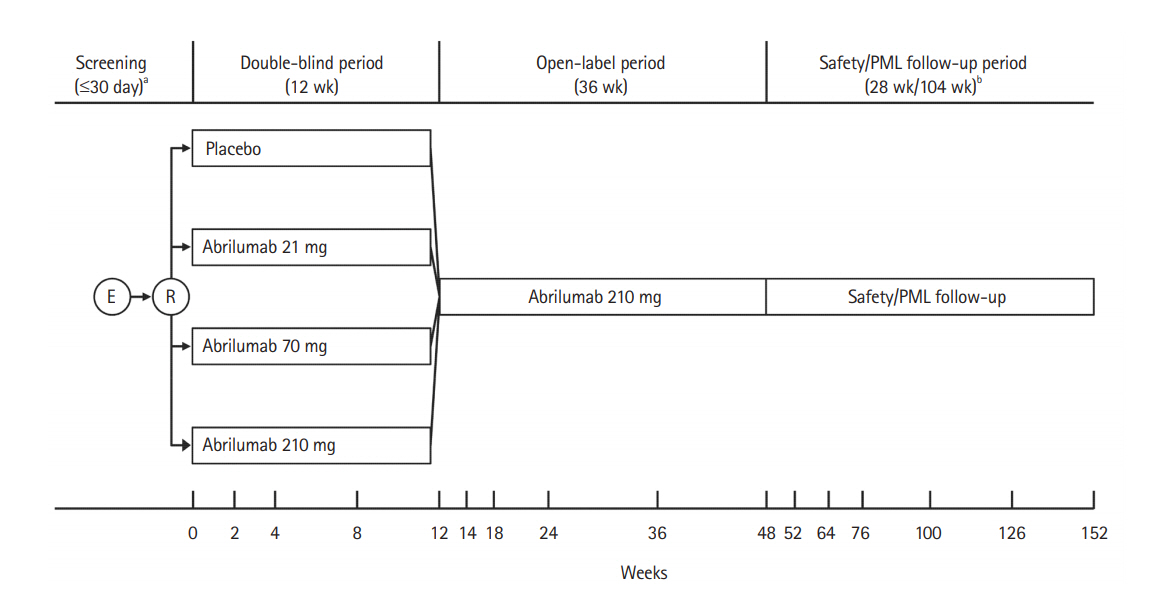
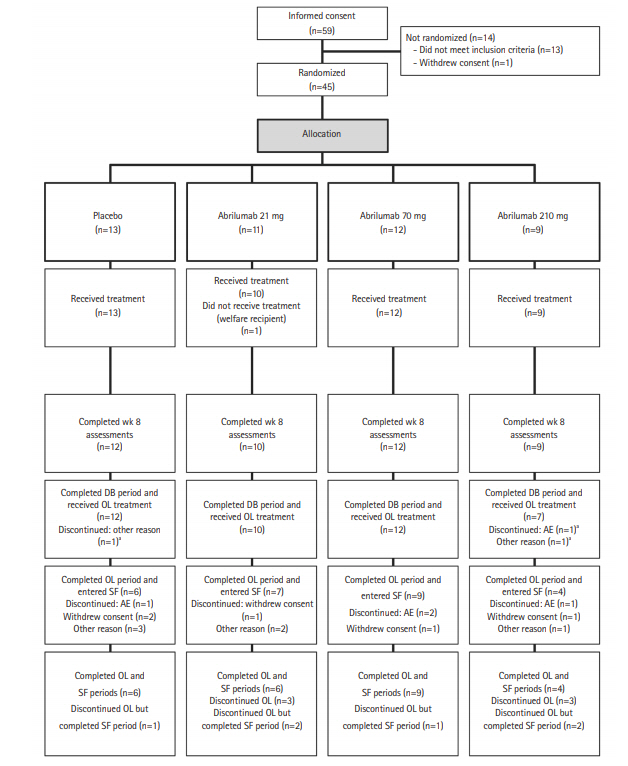
Fig.┬Ā2.
Table┬Ā1.
Table┬Ā2.
| Outcome | Placebo (n=13) |
Abrilumab |
||
|---|---|---|---|---|
| 21 mg (n=10) | 70 mg (n=12) | 210 mg (n=9) | ||
| Remission rate at week 8a | 0 | 1 (10.0) | 2 (16.7) | 1 (11.1) |
| Response rate at week 8b | 7 (53.8) | 3 (30.0) | 6 (50.0) | 6 (66.7) |
| Mucosal healing rate at week 8c | 4 (30.8) | 1 (10.0) | 4 (33.3) | 4 (44.4) |
| Response rate at week 12d | 6 (46.2) | 3 (30.0) | 6 (50.0) | 6 (66.7) |
| Sustained responsee | 4 (30.8) | 3 (30.0) | 3 (25.0) | 4 (44.4) |
Table┬Ā3.
REFERENCES
- TOOLS









