INTRODUCTION
Ulcerative colitis (UC) is a chronic, relapsing, inflammatory disease of the colon characterized by alternating episodes of remission and spontaneous relapse [
1]. Patients with active UC experience significant and clinically meaningful impairment across most aspects of quality of life [
2,
3]. The prevalence of UC in Japan has been steadily increasing from 18.2 in 1991 and 63.6 in 2005 to 121.9 per 100,000 persons in 2013 [
4]; about 170,000 patients were receiving treatment for UC in Japan in 2017 [
5].
Aminosalicylates (5-ASAs) are used as first-line therapy for induction and maintenance of remission in mild to moderately active UC; patients who do not respond to 5-ASAs are treated with corticosteroids (second-line treatment), whereas immunomodulators and biologics are used in moderately to severely active UC [
6]. The introduction of biologics has changed the treatment paradigm for moderately to severely active UC. Currently, adalimumab, infliximab, and golimumab, which target target tumor necrosis factor (TNF)-α, and vedolizumab, which blocks recruitment of immune cells to the gut by targeting α
4β
7 integrin [
7], are approved to treat UC in Japan [
8,
9].
The traditional primary treatment goal in UC has been the induction and maintenance of disease remission. However, the treatment goals for UC have evolved from resolution of UC symptoms to include objective measures such as mucosal healing. Consistent with this trend, the Selecting Therapeutic Targets in Inflammatory Bowel Disease consensus endorses the use of endoscopic outcomes as therapeutic goals in clinical practice [
10].
Population differences may be an important factor in meeting UC treatment goals. For example, differences in phenotypes of UC and susceptibility to UC due to genetic polymorphisms have been reported in different populations [
11,
12]. As such, response to UC treatment may vary in different ethnic populations.
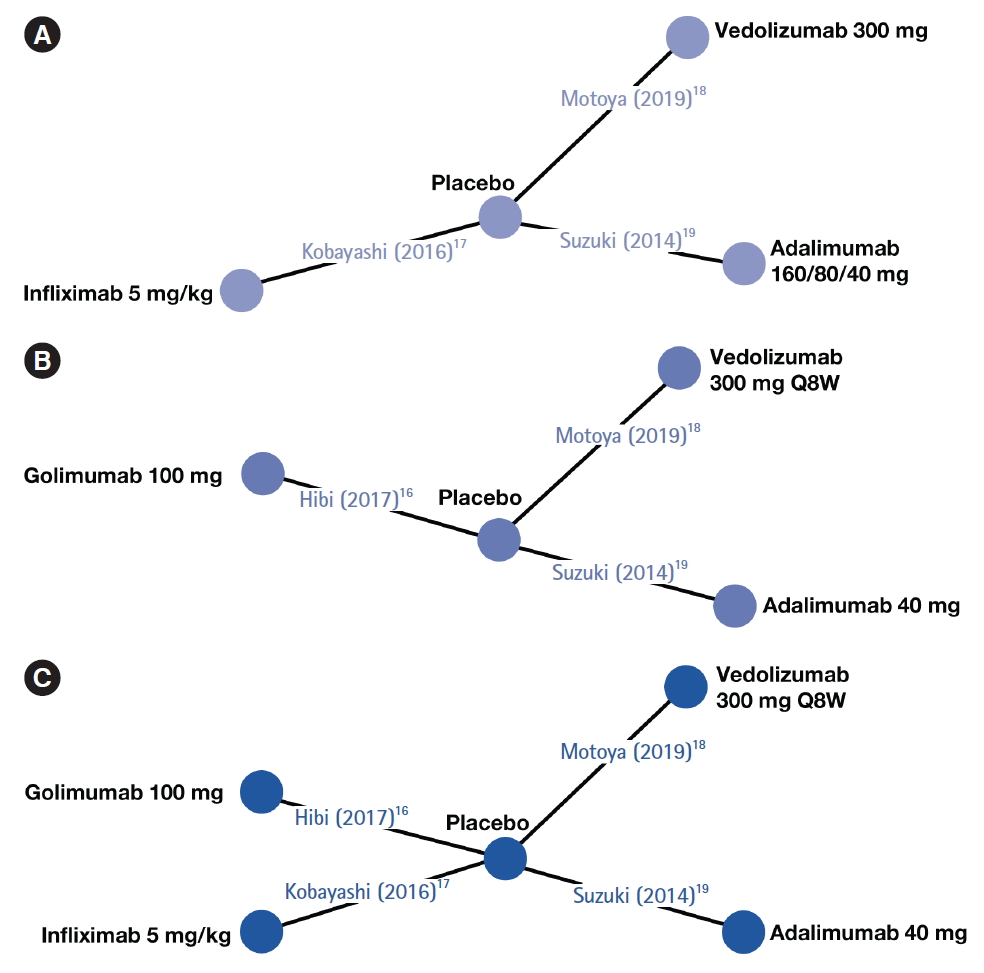
Direct head-to-head trials assessing the relative efficacies of biologics in biologic-naïve Japanese patients are lacking. Assessment of comparative efficacy and safety using network meta-analysis (NMA) can supplement, or compensate for the lack of, relevant randomized controlled trials (RCTs) [
13]. In absence of formal direct head-to-head evidence, these methods can assist policymakers and healthcare professionals in evidence-based decision-making. NMA studies can provide indirect comparisons of treatment efficacy of biologic therapies in moderately to severely active UC and generate useful evidence for judiciously selecting the best choice(s) of treatment. In this study, using data from published RCTs, an NMA was conducted to indirectly compare treatment efficacies of biologics approved in Japan (adalimumab, infliximab, golimumab, and vedolizumab) for treating biologic-naïve patients with moderately to severely active UC.
METHODS
1. Study Selection
A targeted review of the literature was conducted to identity published articles or peer-reviewed, published scientific congress presentations reporting RCTs evaluating approved biologics in biologic-naïve Japanese patients with UC. PubMed and Embase were searched in February 2019 using the following terms: adalimumab, infliximab, golimumab, or vedolizumab, combined with UC. The full search strategies for PubMed and Embase are “(ulcerative colitis [Title] AND (adalimumab [Title/Abstract] OR golimumab [Title/Abstract] OR infliximab [Title/Abstract] OR vedolizumab [Title/Abstract])) AND (randomized [Title/Abstract] OR randomised [Title/Abstract])” and “ulcerative colitis.title. AND (vedolizumab or golimumab or infliximab or adalimumab). abstract. AND (randomized or randomised).abstract.,” respectively. The search was limited to RCTs conducted in human subjects and no language restrictions were applied. Duplicate publications were excluded, and each unique publication identified in the searches was reviewed for relevance by title and abstract. The full text of the selected articles was retrieved and evaluated based on study eligibility criteria. Studies were eligible for inclusion if they were RCTs with more than one treatment arm and assessed the efficacy or safety of biologics for the treatment of biologicnaïve Japanese patients with moderately to severely active UC. Both placebo-controlled trials and active treatment-controlled trials were eligible for inclusion. Only studies that used approved dosing of each drug (as provided in the respective summary of product characteristics) and had a follow-up time of 1 year were included.
2. Outcome Measures and Definitions
Outcome measures of interest in the induction phase were clinical response, clinical remission, and mucosal healing. Induction endpoints were assessed at week 8 for infliximab and adalimumab and at week 10 for vedolizumab. Outcome measures of interest in the maintenance phase were sustained clinical response, sustained clinical remission, and mucosal healing at weeks 30 to 60. Definitions of outcome measures in induction phase and maintenance phase are presented in
Supplementary Table 1.
3. Data Extraction
All data were extracted by 2 independent reviewers and discrepancies were discussed and resolved. Details on the trial’s acronym, first author’s last name, year of publication, study design, and patient baseline characteristics were extracted from each publication. Number of participants and intervention parameters including drug, dosage, and administration were extracted separately for both induction phase and maintenance phase where available. Data were only collected for patients not previously exposed to anti-TNF therapy. Data for pre-specified outcome measures in biologic-naïve patients were extracted for each study at the end of treatment induction and maintenance phases where available. Different dosages of the same treatment were treated as different interventions.
4. Quality Assessment
The risk of bias for each included study was evaluated using the Cochrane Collaboration tool for assessing risk of bias in RCTs [
14]. The following items were evaluated: generation of the allocation sequence (selection bias); concealment of the allocation sequence (selection bias); blinding (detection and performance bias); blinding of participants and personnel and blinding of outcome assessment; incomplete outcome data (attrition bias); selective outcome reporting (reporting bias); other biases (
Supplementary Table 2). For each RCT, each item was evaluated as: low risk of bias, high risk of bias, or unclear risk of bias [
14].
5. Data Synthesis and Analysis
The treatment effect at the end of the induction phase and at 1 year for each biologic was estimated using Bayesian meta-analyses and was transformed into probabilities and odds ratios (ORs) with 95% credible intervals (CrI) using the average placebo results across all trials as a reference. Risk differences were calculated as the differences in the probabilities of each efficacy outcome against placebo. Each of the pre-specified outcome measures was analyzed separately at each relevant time point (induction and maintenance). The analysis assumed that outcome measures were defined similarly between trials.
A binomial likelihood, logit link function, and fixed-effects model were used to account for variability between trials, treatment group, and treatment effect. For direct comparisons, a fixed-effects (as opposed to random-effects) model [
15] was used due to the limited number of studies included in the analysis, which did not allow random-effects terms to be estimated.
Small-study effects and publication bias were not formally assessed, given that each pairwise comparison included a limited number of studies (less than 10). The analysis was performed using OpenBUGS version 3.2.2, following U.K. National Institute for Health and Care Excellence guidance on evidence synthesis methodology.
6. Compliance with Ethical Standards
As part of the original studies used as data sources for the current analyses, all patients provided written informed consent, and the trials were approved by the institutional review board of each participating institution. Because the current post hoc analyses used existing data from published primary studies, additional patient consent was not required.
DISCUSSION
Genetic factors may contribute to UC pathogenesis and susceptibility, including loci identified in the Japanese population [
20]. Population-based factors may also be important in response to treatment. To our knowledge, this is the first NMA to evaluate the relative efficacy of biologics for UC approved in Japan among biologic-naïve Japanese patients with UC. Despite the limited number of studies and the small sample sizes with relevant data in the biologic-naïve Japanese population, our NMA provides insights into relative biologic efficacies in this specific subset of UC patients. Overall, biologic therapies showed varying efficacies during induction and maintenance; however, there was no evidence to suggest substantial differences, and efficacy outcomes were relatively similar among the assessed biologics. Compared with placebo, vedolizumab and infliximab had a similar high likelihood for achieving clinical response, remission, and mucosal healing during induction treatment, whereas golimumab and vedolizumab had the highest likelihood of maintaining these outcomes. During maintenance treatment, in comparison with placebo, golimumab and vedolizumab had the highest likelihood of maintaining efficacy outcomes.
The incidence of adverse events (AEs) associated with the induction treatment of the biologics versus placebo were similar (infliximab, 81.7% vs. 82.7%; adalimumab, 44.4% vs. 46.9%; vedolizumab, 50.0% vs. 52.4%). Compared with placebo, fewer patients had serious AEs, serious infections, or worsening of disease in the induction treatment of infliximab (8.7% vs. 12.5%, 1.0% vs. 1.9%, 7.7% vs. 10.6%, respectively). In contrast, more patients experienced AEs with the maintenance treatment of golimumab (97.0% vs. 71.0%), infliximab (96.2% vs. 90.4%), adalimumab (538 events vs. 273 events), and vedolizumab (87.8% vs. 78.6%). Adalimumab also resulted in a higher number of serious AEs (33 events vs. 14 events), serious infections (8 events vs. 2 events), and worsening of disease (18 events vs. 15 events) than placebo. There were no substantial differences with infliximab in serious AEs (17.3% vs. 18.3%), serious infections (1.0% vs. 1.9%), and worsening of disease (15.4% vs. 17.3%) or with vedolizumab in serious AEs (9.8% vs. 7.1%). Malignancy was reported in 2 patients (pancreatic carcinoma and parathyroid tumor) treated with adalimumab. No available data on malignancy were noted on the other drugs.
In a previous NMA evaluating treatment efficacies in a global population of anti-TNF therapy-naïve UC patients, all analyzed treatments (tofacitinib, adalimumab, golimumab, infliximab, and vedolizumab) were superior to placebo for both induction and maintenance treatment of UC [
21]. In this report, a traditional meta-analysis of outcomes data for each treatment demonstrated similar higher odds of clinical remission, clinical response, and mucosal healing with infliximab (OR: 3.62, 3.97, and 3.05, respectively) followed by vedolizumab (OR: 3.17, 4.26, 2.91, respectively). These results are consistent with results from our indirect treatment comparisons, wherein the induction-phase NMA showed that infliximab treatment was better than adalimumab and golimumab in achieving clinical response, better than adalimumab in achieving clinical remission, and better than adalimumab and golimumab in achieving mucosal healing. No other indirect comparisons reached statistical significance. Because of the differences in study design (patient eligibility for maintenance phase; rerandomization of induction responders), indirect treatment comparisons with maintenance-phase data were not conducted.
Another NMA of global population data in anti-TNF therapy-naïve UC patients also found that all biologics (adalimumab, golimumab, infliximab, and vedolizumab) were more effective at inducing clinical response, remission, and mucosal healing in induction [
22]. In this study, data from both rerandomization trials and straight trials were included in indirect treatment comparisons of maintenance-phase data. Any study design differences were accounted for by assuming the number of responders at end of induction to be equivalent to the number rerandomized. In the maintenance phase, however, only vedolizumab showed significantly better odds across all 3 efficacy outcomes: sustained clinical response, remission, and mucosal healing. In indirect treatment comparisons, vedolizumab showed significantly better durable clinical response than adalimumab, infliximab, and golimumab during maintenance. Vedolizumab also showed a significant improvement in clinical remission over infliximab and significant improvement in mucosal healing over adalimumab.
The recently reported VARSITY trial (NCT02497469) is the first and only published head-to-head RCT comparing the efficacy and safety of 2 biologic therapies, vedolizumab, and adalimumab, in patients with moderately to severely active UC [
23]. The lack of similar comparisons of biologic therapies in head-to-head trials for patients with UC, and in particular patient subpopulations of interest, highlights the importance of performing NMA studies.
Our NMA had several limitations. First, there are few published randomized controlled clinical trials of UC therapies specific to the biologic-naïve Japanese population. Thus, the currently available highest-level clinical evidence base is limited. As a result, comparative efficacy of tofacitinib could not be evaluated as tofacitinib efficacy data in a Japanese subpopulation were based on a small subset of the OCTAVE trials [
24] and thus not powered to detect differences. Second, because of the lack of head-to-head trials, efficacy analyses were based on indirect comparisons. Outcomes of this NMA should be assessed with consideration for the different study designs of each trial. Third, the lack of patient-level data also precluded accounting for differences in patient-related effect modifiers in individual trials (e.g., severity of UC); future analyses should control for such differences. In addition, the relatively small patient sample sizes available for inclusion in the current analyses were a limitation. A safety analysis also could not be conducted due to the small sample sizes and limited available data. Finally, differences in efficacy outcomes in the placebo arms from individual trials may have affected the results of this analysis. For example, the higher efficacy rates in Japanese patients who received placebo in the vedolizumab trial [
18] may have influenced the current NMA results.
In conclusion, among Japanese patients with UC, all biologic therapies evaluated showed superior efficacy relative to placebo, although the degree of efficacy benefit varied between drugs. Vedolizumab and infliximab had the highest likelihood for induction of clinical response, clinical remission, and mucosal healing, whereas golimumab had the highest likelihood of maintaining these outcomes. These results are not generalizable to other patient populations and require confirmation in larger patient populations and real-world settings. Future studies should also evaluate safety in addition to efficacy to determine the relative net benefit of biologic therapies.