 |
 |
- Search
| Intest Res > Volume 20(4); 2022 > Article |
|
Abstract
Background/Aims
Very early-onset inflammatory bowel disease (VEO-IBD), defined as IBD diagnosed in patients younger than 6 years, is a challenge for pediatric gastroenterologists. Although there have been reports regarding VEO-IBD in Western countries, those in Asia are still lacking. This study aimed to investigate the clinical features of Japanese VEO-IBD patients.
Methods
Patients with VEO-IBD diagnosed between 2006 and 2019 were evaluated retrospectively. The disease phenotypes were classified into ulcerative colitis type (UC-type) and CrohnŌĆÖs disease type (CD-type), and the clinical features and courses were compared between the phenotypes.
Results
Overall, 54 VEO-IBD patients (19 patients with UC-type and 35 patients with CD-type) were evaluated. The median age at onset was 18 months. One patient had severe combined immunodeficiency (SCID), and 9 patients had monogenic IBD. Monogenic IBD was more prevalent in the CD-type patients with perianal disease (CD-type (PD)). The age at onset was significantly lower in the CD-type group (P<0.05). The most common initial symptom was bloody stools (70%), followed by diarrhea (63%), weight loss (24%), fever (20%), and perianal disease (20%). Excluding patients with SCID and monogenic IBD, 23 out of 44 patients (52%) required biologics. The biologics were switched in 11 out of 44 patients (25%), and the majority of these patients (82%) were in the CD-type group. Overall, 9 patients (20%) required intestinal resection or ostomy placement.
The incidence of pediatric-onset inflammatory bowel disease (IBD) has been increasing worldwide in the last few decades [1-3]. Very early-onset IBD (VEO-IBD), that is, IBD diagnosed before 6 years of age, accounts for approximately 15% of pediatric IBD cases [4]. Although VEO-IBD was initially considered to have an aggressive disease course with poor response to conventional treatments and higher requirement of surgery [5-7], recent studies have shown that its surgery rate is comparable with that of older children [8]. Another study revealed that the disease severity in VEO-IBD with CrohnŌĆÖs disease (CD) at the time of diagnosis is milder than that in older patients [9]. There have been several reports [6,10-12] on the clinical features and disease course of VEO-IBD from North America and Europe, but similar studies from other countries have been rare [13,14]. A multicenter, cross-sectional study of pediatric IBD involving children up to the age of 17 years [15] and a nationwide survey of VEO-IBD from 1998 to 2008 [16] were carried out in Japan, including some of the patients in this study, but the classification of IBD and the focus of these studies were different. VEO-IBD has more genetic factors for its pathogenesis [17]. VEO-IBD is a heterogeneous group of diseases, and thus, determining its clinical features and disease course across various regions and races would help to elucidate its overall clinical picture. Accumulating adequate data would help improve the diagnostic and treatment accuracy. As such, this study aimed to review the clinical features and disease courses of Japanese pediatric patients with VEO-IBD.
This was a retrospective review of the medical records of a cohort of VEO-IBD patients diagnosed between 2006 and 2019 at the Center for Pediatric IBD of the National Center for Child Health and Development (NCCHD), a tertiary childrenŌĆÖs hospital in Tokyo, Japan. IBD was diagnosed according to the revised Porto criteria with endoscopic and pathological findings. Patients who were initially considered to have IBD-like conditions but were later found to have infectious, eosinophilic gastrointestinal, or non-inflammatory causes were excluded.
VEO-IBD was classified into 2 categories as UC-type (i.e., continuous colonic inflammatory lesion without definite upper gastrointestinal, small bowel, or perianal involvement) and CD-type (i.e., intestinal inflammatory lesion not consistent with UC-type). This was because a certain number of VEO-IBD patients cannot be diagnosed with conventional UC, CD, or IBD-unclassified. This classification covers all patients with VEO-IBD, including those with monogenic IBD. Patients with perianal disease were classified as having CD-type (PD). Perianal disease was defined in patients who had a fistula, anal canal ulcer, or abscess.
The disease location and severity were categorized based on the Paris classification [18]. The classifications of UC and CD were defined as UC-type and CD-type, respectively. Thus, in these evaluations, patients for which total gastrointestinal tract evaluation could not be performed were excluded from the CD-type. To diagnose inborn errors of immunity, the results of Sanger sequencing, insurance-covered targeted panel sequencing (IL10, IL10RA, IL10RB, FOXP3, IL2RA, STAT1, TNFAIP3, MALT1, CTLA4, XIAP, STAT5B, TTC7A, SLCO2A1, LRBA, IL21, WAS, CYBA, CYBB, NCF2, and NCF4), whole-exome sequencing, and flow cytometry analysis performed at the discretion of the caring physicians were reviewed. Those diagnosed or suspected to have monogenic IBD were included in this study; however, patients with severe combined immunodeficiency (SCID) and monogenic IBD that were treated with bone marrow transplantation (BMT) were excluded from the analysis for medical treatment.
Continuous data were described as medians (ranges), while discrete data were described as percentages. Fisher exact test was used to evaluate the differences in sex, gastrointestinal symptoms, extraintestinal manifestations at the onset, and use of biologics between the UC-type and CD-type groups. The Mann-Whitney U test was used to assess the differences in age at onset and diagnosis between the 2 groups. All statistical analyses were performed using the EZR software system (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [19]. Statistical significance was set at P<0.05.
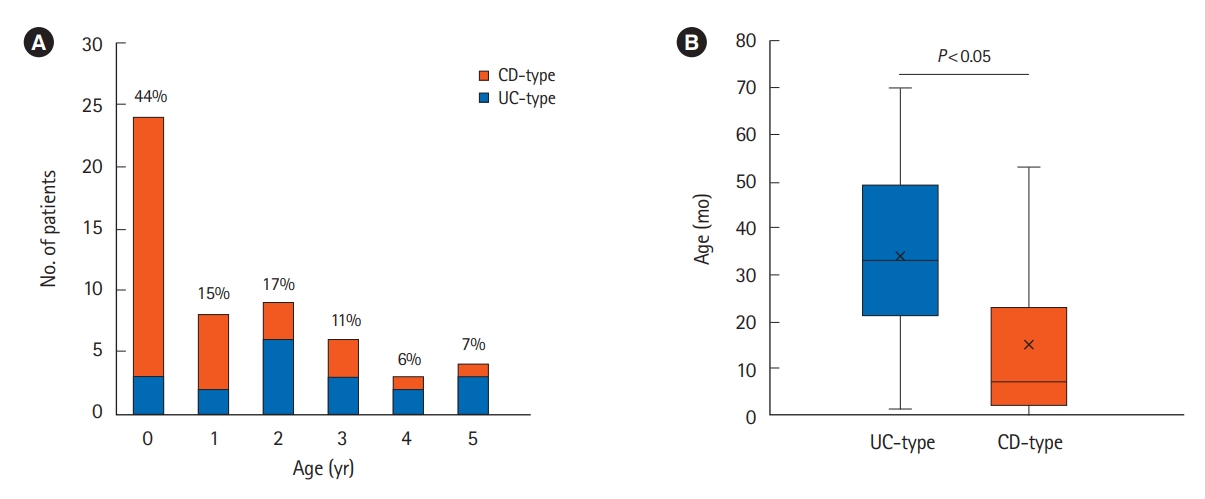
In total, 54 patients with VEO-IBD (19 patients with UC-type and 35 patients with CD-type) were evaluated. Twelve patients with CD-type had perianal disease (CD-type (PD)). The patient characteristics are shown in Table 1. The median ages at onset and diagnosis of VEO-IBD were 18 months (range, 0-70 months) and 28 months (range, 2-70 months), respectively. The median follow-up period was 4.3 years (range, 0.5-13.5 years). Overall, 24 patients (44%) developed IBD before the age of 1 year (Fig. 1), and 33 patients (61%) were diagnosed before the age of 3 years. The age at onset and diagnosis was significantly younger in the CD-type group, particularly those with CD-type (PD), than in the UC-type group (P<0.05).
The initial symptoms at disease onset are shown in Table 1. All patients in the UC-type group had hematochezia, but only approximately half of the CD-type group had the same symptom (P<0.05). Meanwhile, 11 patients in the CD-type group (31%) had perianal disease at onset. Vomiting was observed in 1 patient in the UC-type group (5%) and 1 patient in the CD-type group (3%) at the onset. One patient with UC-type had overlap syndromes of primary sclerosing cholangitis and autoimmune hepatitis.
Colonoscopy was performed in all 54 patients (100%), while esophagogastroduodenoscopy was performed in 49 patients (91%). Evaluation of small intestinal lesions was performed in 34 patients (63%), including small intestinal capsule endoscopy in 13 patients (24%), small bowel follow-through in 16 patients (30%), and contrast-enhanced computed tomography in 12 patients (22%). In total, 41 patients (76%) underwent genetic testing; 10 patients (19%), targeted panel sequencing; 25 patients (46%), whole-exome sequencing; and 7 patients (13%), Sanger sequencing. Of them, 9 out of 41 (22%) were diagnosed with monogenic IBD, including 6 patients with chronic granulomatous disease (CGD); 1 patient, interleukin-10 signaling defect; 1 patient, XIAP deficiency; and 1 patient, Wiskott-Aldrich syndrome. All these conditions were categorized as CD-type, and 8 of 9 monogenic IBD patients had PD.
Monogenic IBD was significantly more prevalent in the CDtype group (P<0.05). One patient with CD-type was clinically diagnosed with SCID on immunological and genetic analyses. This patient developed Pneumocystis jiroveci pneumonia at 5 months of age. Immunological assessment demonstrated a marked decrease of T cells and B cells (normal NK) in peripheral blood; low T-cell receptor excision circles, indicating T cell lymphopenia [20]; and low kappa-deleting recombination excision circles, indicating B cell lymphopenia [21]. However, no mutations were identified in the disease-related genes (RAG1, RAG2, DCLRE1C, PRKDC, NHEJ1, LIG4, ADA, AK2, and RAC2). Diarrhea was observed at 2 months of age, and infectious enteritis was ruled out by stool culture. Endoscopic and histological evaluations revealed IBD at 9 months of age.
Table 2 shows the extent and severity of UC-type, as well as the disease location and clinical behavior of CD-type. In the UC-type group, 15 patients (79%) had pancolitis at diagnosis. Only 4 patients (21%) were classified to have S1 disease (i.e., a pediatric UC activity index score of > 65). In the CD-type group, 23 patients (66%) were evaluated for the involvement of the total gastrointestinal tract, including the small intestine, and L2 (colonic involvement only) was the most common disease location. Upper gastrointestinal lesions (L4a, L4b, and L4a+L4b) were found in 11 patients (48%), and perianal disease was found in 10 patients (43%). There were 2 patients (9%) who had a stenotic lesion classified as B2 at the time of diagnosis.
In 2 patients, the diagnosis of severe UC-type was changed to CD-type during the follow-up period. One patient with severe UC-type who underwent total colectomy with ileostomy placement developed ileal lesions with steroid-responsible diarrhea. Another patient with refractory UC-type developed jejunal and ileal lesions 3 years after the diagnosis of UC-type.
Table 3 shows the cumulative exposure to treatments. Patients with SCID and monogenic IBD were excluded from this analysis. 5-Aminosalicylic acid (5-ASA) was used in almost all patients, and azathioprine (AZA) or 6-mercaptopurine was used in 32 patients (73%). Steroids were used for induction therapy in 29 patients (66%). During the follow-up period, biologics were used in 23 patients (52%), with infliximab being the most commonly used. There were 11 patients (25%) who received a second or third biologic. There was no difference in biologics use between the UC-type and CD-type groups, but the proportion of patients that required a biologics switch was higher in the CD-type group, although the difference was not significant. Overall, 6 UC-type patients (32%) and 5 CD-type patients (20%) achieved and remained in remission, requiring only 5-ASA and partial enteral nutrition. In addition, 5-ASA and AZA were reduced or discontinued during follow-up in 1 UC-type patient (5%) and 4 CD-type patients (16%) as these patients were on long-term remission. All but 1 monogenic IBD patient and 1 SCID patient underwent BMT, and their gastrointestinal symptoms improved significantly. One CGD patient is scheduled for BMT.
There were 10 patients (19%), including 1 with monogenic IBD, who underwent intestinal-related surgery. Total colectomy was performed in 4 UC-type patients. Meanwhile, 5 CD-type patients required ostomy placement to spare the inflamed intestinal tract. One CD-type patient with an uncontrolled intractable disease, even after a diverting ileostomy placement underwent total colectomy. In addition, 2 CD-type patients (1 with perforation and the other with bleeding) required laparotomy for post-endoscopic complications.
Data on the clinical characteristics of VEO-IBD in Japanese patients are scarce. In this study, approximately half of the VEO-IBD patients had disease onset before the age of 3 years, and most of the patients were diagnosed with CD-type. The severity of VEO-IBD varies among patients, and thus, treatment should be individualized. In a Japanese nationwide survey of VEO-IBD, which had a 92% response rate, 56%, 21%, 8%, 3%, and 12% had UC, CD, IBD-unclassified, Beh├¦etŌĆÖs disease, and immunodeficiency-associated enteritis, respectively [16]. Further, half of the patients were diagnosed under the age of 3 years, which is consistent with the findings in our study. Compared with a North American cohort of VEO-IBD in which 26% of VEO-IBD patients were diagnosed under the age of 3 years [11], Japanese VEO-IBD patients appear to have a younger onset. This may suggest more genetic components for their disease origin. A larger nationwide cohort with more genetic data would enable a better comparison across different regions worldwide.
In our study, all UC-type patients presented with bloody stools, but only 21% of the patients had severe disease (S1) at diagnosis. One of the factors for the relatively lower S1 rate may be the earlier coloscopy and diagnosis with early detection of bloody stools in this age group. Nevertheless, most of the patients already had extended disease at the time of diagnosis. In a Japanese registry study [15], 88% of patients aged 0-5 years had E4 disease. Meanwhile, in a single-center study from North America [11], E4 patients accounted for 67% of those with VEO-IBD/UC. Extended disease is one of the characteristics of UC-type/UC in VEO-IBD worldwide. In this study, although the proportion of patients with S1 disease at diagnosis was not high, biologics were required in 47% of the UC-type patients during the follow-up. In addition, 21% of UC-type patients required a colectomy or diverting ileostomy. Accelerated stepup should be considered in UC-type patients who do not respond to conventional treatment for UC.
More than half of our patients had CD-type, and 26% of them were found to have monogenic IBD. The CD-type group included a high proportion of L2 patients. This is in line with reports from a registry study in Japan [15] and a single-center study in the United States [11] that showed that the proportion of L2 patients is higher in VEO-IBD patients than in IBD patients of older ages. Limited modalities to evaluate small bowel lesions in this small group of children may have contributed to the high number of L2 lesions. In the current study, 31% of CD-type patients have perianal disease, which is a higher rate than in North American patients with VEO-CD [11]. A Japanese multicenter pediatric CD registry also showed significantly higher rates of PD modifier than in the European registry [15].
With respect to treatment, the number of patients who received 2 or more biologics was higher among those with CD-type without monogenic IBD than in those with UC-type, and 20% of patients with CD-type without monogenic IBD required ostomy placement. Meanwhile, some patients did not require steroids, immunomodulators, or biologics, and some children who initially required steroids or biologics maintained long-term remission with only 5-ASA. Mild cases responding to conventional therapy have been described in previous studies [9,10,22]. Collectively, these findings support that the severity of CD-type varies among patients. Accordingly, genetic and immunological testing are essential for the proper treatment of monogenic IBD and primary immunodeficiency-associated IBD, especially in patients with CD-type onset before the age of 3 years.
This study has some limitations. First, this was a retrospective single-center study conducted at a pediatric IBD center, and the disease severity of this cohort may not reflect that in the general population. In addition, the possibility of selection bias could not be ruled out. Second, small bowel imaging studies were not performed in some patients, especially in patients diagnosed with CGD before IBD and in patients in whom capsule endoscopy is not feasible. Other limitations include the difference in the follow-up periods among the patients and in the available genetic testing or standardized treatment. A multicenter registry study for VEO-IBD would further elucidate this challenging condition.
In conclusion, this study showed that half of VEO-IBD cases develop before the age of 3 years, and VEO-IBD can present with a wide range of disease severity from mild to severe cases. CD-type, especially that which develops at an earlier age, is characterized by many intractable cases requiring biologics. Monogenic IBD is highly common among CD-type (PD) patients. Given the heterogeneous nature of VEO-IBD, early diagnosis with genetic and immunological testing is crucial.
ADDITIONAL INFORMATION
Funding Source
This work was supported in part by a Grant-in-Aid for the National Center for Child Health and Development from the Ministry of Health, Labor and Welfare, Japan (2019A-3 to Arai K).
Author Contribution
Conceptualization: Usami M, Takeuchi I, Arai K. Data curation: Usami M, Kyodo R, Hirano Y, Kashiwagi K, Fujikawa H, Shimizu H. Formal analysis: Usami M. Funding acquisition: Arai K. Methodology: Usami M, Takeuchi I, Arai K. Supervision: Kawai T, Arai K. Writing - original draft: Usami M. Writing - review & editing: Takeuchi I, Kawai T, Arai K. Approval of final manuscript: all authors.
Fig.┬Ā1.
Age at onset of very early-onset inflammatory bowel disease. (A) Age distribution at onset. (B) Comparison of age at onset between the ulcerative colitis (UC)-type and CrohnŌĆÖs disease (CD)-type groups. The Mann-Whitney U test is used to assess the differences between the UC-type and CD-type groups.
Table┬Ā1.
Patient Characteristics
Table┬Ā2.
Inflammatory Bowel Disease Phenotype at Diagnosis (Paris Classification)
Table┬Ā3.
Cumulative Exposure to Treatments
REFERENCES
1. Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis 2011;17:423-439.


2. Ishige T, Tomomasa T, Takebayashi T, et al. Inflammatory bowel disease in children: epidemiological analysis of the nationwide IBD registry in Japan. J Gastroenterol 2010;45:911-917.



3. Mart├Łn-de-Carpi J, Rodr├Łguez A, Ramos E, et al. Increasing incidence of pediatric inflammatory bowel disease in Spain (1996-2009): the SPIRIT Registry. Inflamm Bowel Dis 2013;19:73-80.

4. Heyman MB, Kirschner BS, Gold BD, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr 2005;146:35-40.


5. Kammermeier J, Dziubak R, Pescarin M, et al. Phenotypic and genotypic characterisation of inflammatory bowel disease presenting before the age of 2 years. J Crohns Colitis 2017;11:60-69.



6. Aloi M, Lionetti P, Barabino A, et al. Phenotype and disease course of early-onset pediatric inflammatory bowel disease. Inflamm Bowel Dis 2014;20:597-605.


7. Ledder O, Catto-Smith AG, Oliver MR, Alex G, Cameron DJ, Hardikar W. Clinical patterns and outcome of early-onset inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2014;59:562-564.


8. Kerur B, Machan JT, Shapiro JM, et al. Biologics delay progression of CrohnŌĆÖs disease, but not early surgery, in children. Clin Gastroenterol Hepatol 2018;16:1467-1473.


9. Oliva-Hemker M, Hutfless S, Al Kazzi ES, et al. Clinical presentation and five-year therapeutic management of very early-onset inflammatory bowel disease in a Large North American Cohort. J Pediatr 2015;167:527-532.


10. Kelsen JR, Conrad MA, Dawany N, et al. The unique disease course of children with very early onset-inflammatory bowel disease. Inflamm Bowel Dis 2020;26:909-918.




11. Kerur B, Benchimol EI, Fiedler K, et al. natural history ofvery early onset inflammatory bowel disease in North America: a retrospective cohort study. Inflamm Bowel Dis 2021;27:295-302.



12. Bequet E, Sarter H, Fumery M, et al. Incidence and phenotype at diagnosis of very-early-onset compared with later-onset paediatric inflammatory bowel disease: a population-based study [1988-2011]. J Crohns Colitis 2017;11:519-526.


13. Al-Hussaini A, El Mouzan M, Hasosah M, et al. Clinical pattern of early-onset inflammatory bowel disease in Saudi Arabia: a multicenter national study. Inflamm Bowel Dis 2016;22:1961-1970.

14. Fang YH, Luo YY, Yu JD, Lou JG, Chen J. Phenotypic and genotypic characterization of inflammatory bowel disease in children under six years of age in China. World J Gastroenterol 2018;24:1035-1045.



15. Arai K, Kunisaki R, Kakuta F, et al. Phenotypic characteristics of pediatric inflammatory bowel disease in Japan: results from a multicenter registry. Intest Res 2020;18:412-420.




16. Kudo T, Arai K, Uchida K, et al. Very early-onset inflammatory bowel disease in Japan: a nationwide survey. J Gastroenterol Hepatol 2021;36:151-155.



17. Okou DT, Kugathasan S. Role of genetics in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2014;20:1878-1884.



18. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17:1314-1321.

19. Kanda Y. Investigation of the freely available easy-to-use software ŌĆśEZRŌĆÖ for medical statistics. Bone Marrow Transplant 2013;48:452-458.




20. van der Spek J, Groenwold RH, van der Burg M, van Montfrans JM. TREC based newborn screening for severe combined immunodeficiency disease: a systematic review. J Clin Immunol 2015;35:416-430.




- TOOLS








