|
 |
- Search
| Intest Res > Volume 21(4); 2023 > Article |
|
Abstract
Background/Aims
Methods
Results
ADDITIONAL INFORMATION
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Author Contribution
Conceptualization: Takahiro N, Sadahiro F, Daisuke M, Satoshi S, Fumihito H. Data curation: all authors. Formal analysis: Takahiro N, Daisuke M, Satoshi S, Fumihito H. Investigation: all authors. Methodology: Sadahiro F, Daisuke M, Satoshi S, Fumihito H. Project administration: Takahiro N. Supervision: Satoshi S, Fumihito H. Writing - original draft: Takahiro N. Writing - review & editing: Takahiro N, Satoshi S, Fumihito H. Approval of final manuscript: all authors.
Fig. 1.
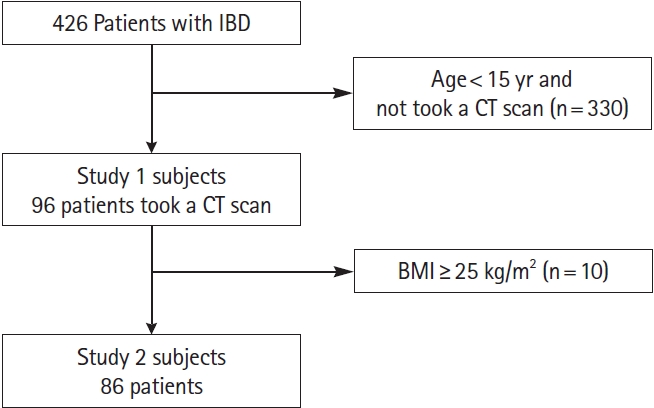
Fig. 2.

Fig. 3.

Fig. 4.

Fig. 5.

Table 1.
| Without NAFLD | With NAFLD | All | |
|---|---|---|---|
| No. of patients | 68 (79.1) | 18 (20.9) | 86 |
| BMI | |||
| < 18.5 kg/m2 | 16 (66.7) | 8 (33.3) | 24 |
| 18.5 to < 25 kg/m2 | 52 (83.9) | 10 (16.1) | 62 |
Table 2.
| Characteristic | Without NAFLD | With NAFLD | P-value |
|---|---|---|---|
| No. of patients | 68 | 18 | |
| Age (yr) | 32 (15-81) | 24 (16-74) | 0.208 |
| Sex (male/female) | 38/30 | 12/6 | 0.409 |
| Drinking history | 8 (11.8)a | - | - |
| Smoking history | 23 (33.8) | 1 (5.6) | 0.017 |
| Height (cm) | 163.0 (143.0-186.0) | 163.6 (153.5-179.7) | 0.364 |
| Body weight (kg) | 51.6 (34.5-83.0) | 50.0 (38.5-68.9) | 0.535 |
| Body mass index (kg/m2) | 20.0 (12.0-24.9) | 18.8 (14.4-23.8) | 0.075 |
| CD/UC | 31/37 | 10/8 | 0.451 |
| CD (ileal/colonic/ileocolonic/unknown) | 5/9/14/3 | 0/1/7/2 | 0.044b |
| UC (proctitis/left-sided/extensive/unknown) | 3/6/19/9 | 0/0/7/1 | 0.030c |
| Use of enteral nutrition | 14 (21.2) | 2 (11.1) | 0.333 |
| Use of steroids | 18 (26.9) | 2 (11.1) | 0.162 |
| Use of biologics | 33 (49.3) | 10 (55.6) | 0.635 |
| Untreated | 6 (8.8) | 4 (22.2) | 0.141 |
| Time from diagnosis to CT scan (mo) | 12 (0-191) | 1 (0-137) | 0.058 |
| White blood cells ( × 103/μL) | 7.3 (3.3-19.1) | 8.4 (3.6-19.1) | 0.061 |
| Lymphocyte count (/μL) | 1,522 (244-3,168) | 1,091 (598-1,984) | 0.063 |
| Platelets ( × 103/μL) | 332 (179-585) | 397 (182-762) | 0.018 |
| C-reactive protein (mg/dL) | 1.70 (0.00-38.56) | 6.86 (0.58-25.42) | < 0.001 |
| Albumin (g/dL) | 3.5 (2.0-4.9) | 2.9 (1.6-4.3) | 0.005 |
| Total bilirubin (mg/dL) | 0.5 (0.2-1.7) | 0.4 (0.3-2.1) | 0.687 |
| Aspartate aminotransferase (U/L) | 17 (9-126) | 18 (12-89) | 0.262 |
| Alanine aminotransferase (U/L) | 12 (5-388) | 20 (7-92) | 0.018 |
| Lactate dehydrogenase (U/L) | 151 (97-327) | 130 (64-300) | 0.287 |
| Alkaline phosphatase (U/L) | 216 (120-772) | 226 (119-3,331) | 0.628 |
| γ-Glutamyl transpeptidase (U/L) | 21 (9-342) | 32 (14-641) | 0.034 |
| Triglycerides (mg/dL) | 80 (35-323) | 77 (53-149) | 0.930 |
| Total cholesterol (mg/dL) | 150 (82-274) | 130 (76-172) | 0.010 |
| Prognostic nutritional index | 43.2 (25.3-57.6) | 35.8 (23.1-48.0) | 0.002 |
| Diabetes mellitus | 3 (4.4) | 0 | 0.364 |
| Muscle mass at the L3 level | |||
| Male | 46.3 (18.0-68.0) | 40.5 (29.2-59.7) | 0.430 |
| Female | 35.6 (27.2-43.1) | 31.9 (26.3-36.5) | 0.161 |
| Psoas muscle index | |||
| Male | 5.41 (1.82-9.72) | 4.89 (3.34-9.75) | 0.411 |
| Female | 3.61 (2.23-5.87) | 3.53 (2.65-4.61) | 0.503 |
REFERENCES
- TOOLS