 |
 |
- Search
| Intest Res > Volume 21(4); 2023 > Article |
|
Abstract
Background/Aims
Methods
Results
ADDITIONAL INFORMATION
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
Sood A is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions
Conceptualization: Midha V, Sood A. Data curation: Singh A, Narang V, Kedia S, Dhoble P, Kahlon BK, Dhaliwal AS, Tripathi A, Kalra S, Bansal N, Banerjee R, Desai D, Dutta U, Ahuja V. Formal analysis: Singh A, Narang V, Bansal N. Investigation: Singh A, Midha V, Sood A. Methodology: Singh A, Midha V, Sood A. Project administration: Midha V, Sood A. Resources: Singh A, Midha V, Narang V, Dhoble P, Jain NP, Desai D, Sood A. Software: Singh A, Kahlon BK, Bansal N. Supervision: Midha V, Sood A. Visualization: Singh A, Kedia S, Mahajan R, Jain NP, Bansal N, Desai D, Ahuja V, Sood A. Writing - original draft: Singh A, Narang V. Writing - review & editing: all authors. Approval of final manuscript: all authors.
Fig.┬Ā1.
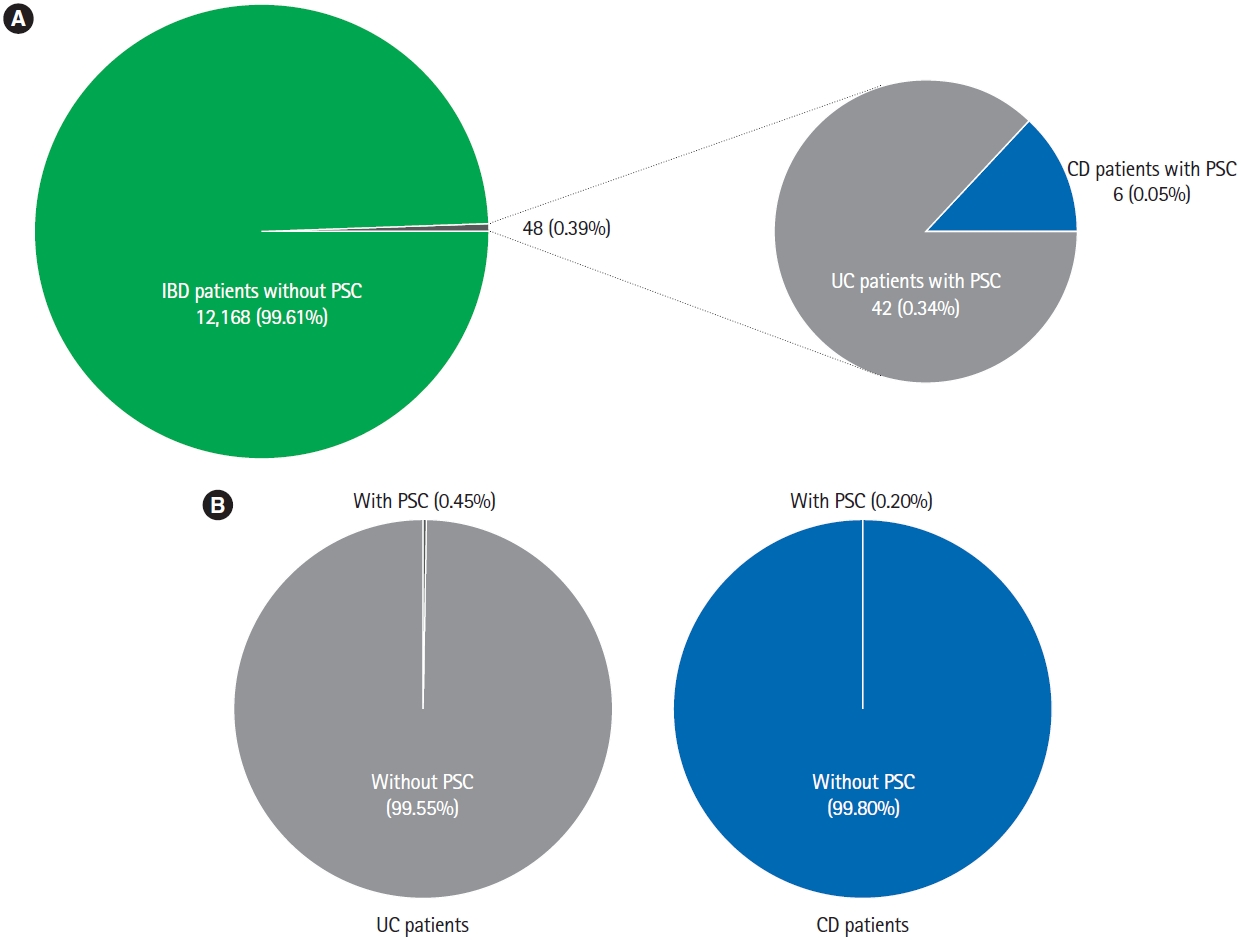
Fig.┬Ā2.

Table┬Ā1.
| Characteristics | Value (n = 48) |
|---|---|
| Male sex | 27 (56.25) |
| Age at diagnosis of IBD (yr) | 33.67 ┬▒ 12.16 |
| Age at diagnosis of PSC (yr) | 37.27 ┬▒ 12.78 |
| Follow up (mo) | 66 (27-129) |
| Current or former smoker | 3 (6.25) |
| PSC diagnosed before IBD | 4 (8.33) |
| IBD diagnosed before PSC | 34 (70.83) |
| IBD and PSC diagnosed simultaneously | 10 (20.83) |
| Interval between diagnosis of IBD and PSC (mo) | 43.25 ┬▒ 74.66 |
| Type of IBD | |
| ŌĆāUlcerative colitis | 42 (87.5) |
| ŌĆāCrohnŌĆÖs disease | 6 (12.5) |
| Disease extent (ulcerative colitis) | |
| ŌĆāProctitis | - |
| ŌĆāLeft sided colitis | 13 (30.95) |
| ŌĆāPancolitis | 29 (69.05) |
| Mayo Clinic score at diagnosis of IBD | 5.26 ┬▒ 2.41 |
| Endoscopic Mayo Clinic score at diagnosis of IBD | 1.64 ┬▒ 0.62 |
| Disease classification (CrohnŌĆÖs disease) | |
| ŌĆāAge at diagnosis | |
| ŌĆāŌĆā< 17 yr | - |
| ŌĆāŌĆā17-40 yr | 4 (66.66) |
| ŌĆāŌĆā> 40 yr | 2 (33.33) |
| ŌĆāDisease location | |
| ŌĆāŌĆāIleal | 1 (16.66) |
| ŌĆāŌĆāColonic | 3 (50.00) |
| ŌĆāŌĆāIleo-colonic | 2 (33.33) |
| ŌĆāDisease behavior | |
| ŌĆāŌĆāNon-stricturing, non-penetrating | 5 (83.33) |
| ŌĆāŌĆāStricturing | 1 (16.66) |
| ŌĆāPenetrating | - |
| Perianal disease | Nil |
| Harvey Bradshaw Index at diagnosis of IBD | 5.17 ┬▒ 1.33 |
| Patients with other extraintestinal manifestationsa | 9 (18.75) |
| ŌĆāArthritis | 5 (10.41) |
| ŌĆāOcular | 4 (8.33) |
| ŌĆāGall stones | 1 (2.08) |
| ŌĆāErythema nodosum | 1 (2.08) |
| Family history of IBD | 1 (2.08) |
| Previous appendectomy | 4 (8.33) |
| Previous exposure to anti-tubercular therapy | 7 (14.58) |
| Treatment for IBD | |
| ŌĆā5-Aminosalicylates | 39 (81.25) |
| ŌĆāThiopurines | 14 (29.16) |
| ŌĆāAnti-TNF | 1 (2.08) |
| ŌĆāCorticosteroids | 20 (41.66) |








