 |
 |
- Search
| Intest Res > Volume 21(3); 2023 > Article |
|
Abstract
Background/Aims
Methods
Results
ADDITIONAL INFORMATION
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
Kanai T received joint research support from Ezaki Glico Co. Ltd., Miyarisan Pharma Corporation; grant support from AbbVie GK, Mochida Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Daiichi-Sankyo Co. Ltd., Mitsubishi Tanabe Pharma Corporation, JIMRO Co. Ltd., ZERIA Pharmaceutical Co. Ltd., Sumitomo Dainippon Pharma Co., Ltd., Bayer Yakuhin, Ltd., EA Pharma Co. Ltd.; and lecture fees from Mitsubishi Tanabe Pharma Corporation, Miyairisan Pharma Corporation, Takeda Pharmaceutical Co. Ltd., Mylan Inc., EA Pharma Co. Ltd., AbbVie GK. Ogata H received joint research support from AbbVie GK, Mochida Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co. Ltd., and lecture fees from Takeda Pharmaceutical Co., Ltd. No other potential conflicts of interest relevant to this article were reported.
Author Contribution
Conceptualization: Aoki Y, Kiyohara H, Mikami Y, Nanki K, Kawaguchi T. Data curation: Aoki Y. Formal analysis: Aoki Y, Kiyohara H. Investigation: Aoki Y, Kiyohara H. Methodology: Aoki Y, Kiyohara H, Mikami Y, Nanki K, Kawaguchi T, Yoshimatsu Y, Sugimoto S, Sujino T, Takabayashi K. Project administration: Kiyohara H, Mikami Y. Resources: Kanai T. Software: Aoki Y, Kiyohara H. Supervision: Kiyohara H, Kanai T. Validation: Aoki Y. Visualization: Aoki Y, Kiyohara H. Writing - original draft: Aoki Y. Writing - review & editing: all author. Approval of final manuscript: all author.
Supplementary Material
Fig. 1.
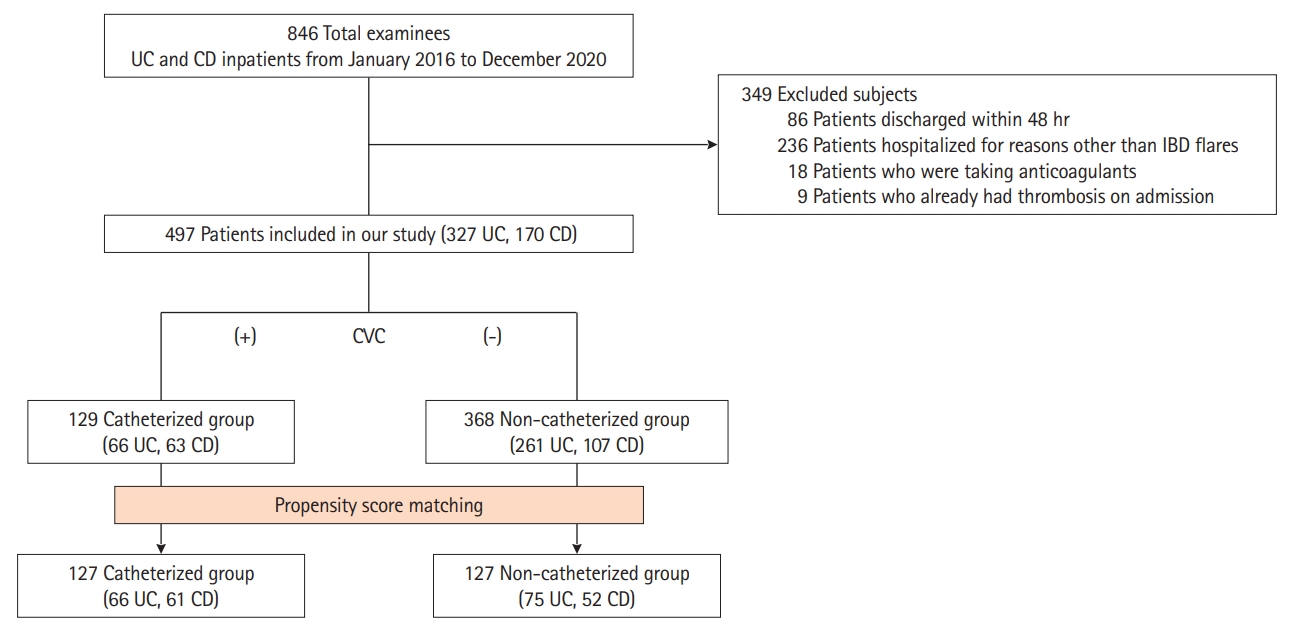
Fig. 2.
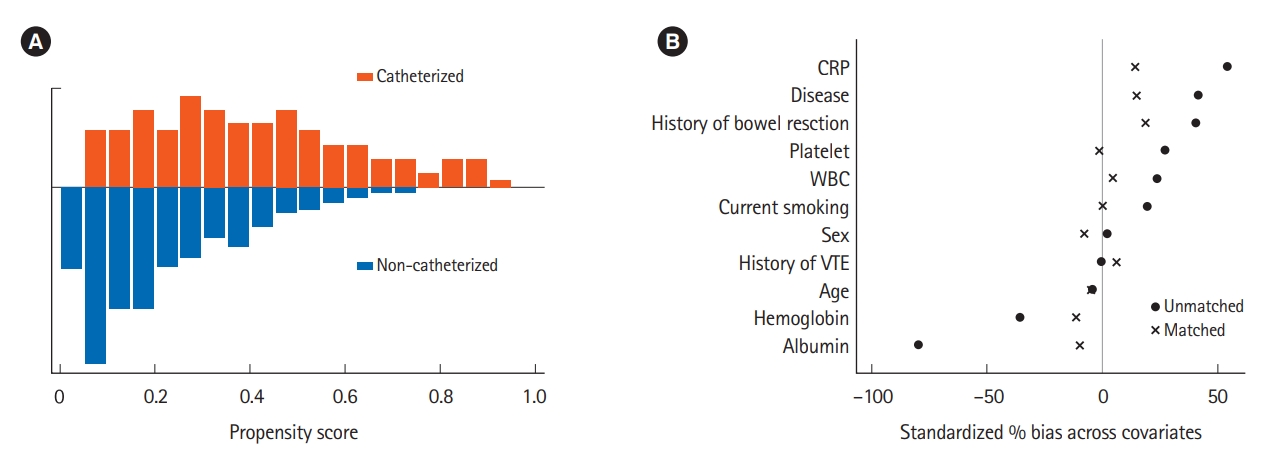
Table 1.
| Characteristic | Catheterized (n = 129) | Non-catheterized (n = 368) | P-value | |
|---|---|---|---|---|
| Male sex | 70 (54.3) | 203 (55.2) | 0.918a | |
| Age (yr) | 39 (30-47) | 38 (26-50) | 0.768b | |
| Body mass index (kg/m2) | 19.3 (17.6-21.7) | 19.9 (18.2-22.2) | 0.149b | |
| Current smoking | 32 (24.8) | 62 (16.9) | 0.051a | |
| Disease and extensionc | ||||
| Ulcerative colitis | 66 (51.2) | 261 (70.9) | 0.592a | |
| E1 | 0 | 3 | - | |
| E2 | 4 | 26 | - | |
| E3 | 62 | 232 | - | |
| Crohn’s disease | 63 (48.8) | 107 (29.1) | 0.126a | |
| L1 | 16 | 19 | - | |
| L2 | 4 | 17 | - | |
| L3 | 43 | 71 | - | |
| Disease duration (yr) | 8.0 (3.0-17.0) | 7.0 (2.0-18.0) | 0.362b | |
| Disease activity | ||||
| Partial Mayo score | 7.0 (6.0-8.0) | 6.0 (5.0-7.0) | 0.011b | |
| Harvey-Bradshaw Index | 7.5 (5.0-10.0) | 6.0 (4.0-8.0) | 0.028b | |
| Missing data | 38 (29.5) | 72 (19.6) | - | |
| Past history of VTE | 2 (1.6) | 6 (1.6) | 1.000a | |
| Past history of bowel resection | 55 (42.6) | 88 (24.0) | < 0.001a | |
| Oral contraceptive pills | 1 (0.8) | 4 (1.1) | 1.000a | |
| Treatment during admission | ||||
| 5-ASA | 61 (47.3) | 238 (64.7) | 0.001a | |
| Azathioprine/6-MP | 31 (24.0) | 123 (33.4) | 0.047a | |
| Systemic corticosteroid | 40 (31.0) | 153 (41.6) | 0.036a | |
| Anti-TNF-α agent | 42 (32.6) | 79 (21.5) | 0.017a | |
| Vedolizumab | 10 (7.8) | 18 (4.9) | 0.266a | |
| Ustekinumab | 5 (3.9) | 4 (1.1) | 0.055a | |
| Tofacitinib | 4 (3.1) | 12 (3.3) | 1.000a | |
| Tacrolimus | 23 (17.8) | 69 (18.8) | 0.895a | |
| Cyclosporin A | 17 (13.2) | 6 (1.6) | < 0.001a | |
| Laboratory data on admission | ||||
| White blood cells (/µL) | 8,800 (6,100-12,300) | 8,000 (6,000-11,000) | 0.098b | |
| Hemoglobin (g/dL) | 11.5 (9.9-12.7) | 12.4 (10.7-13.7) | < 0.001b | |
| Platelet (× 104/µL) | 35.6 (29.3-45.2) | 31.7 (25.1-40.6) | 0.002b | |
| C-reactive protein (mg/dL) | 6.0 (2.7-11.4) | 2.1 (0.0-6.3) | < 0.001b | |
| Albumin (g/dL) | 3.0 (2.7-3.5) | 3.5 (3.1-4.0) | < 0.001b | |
| D-dimer (µg/mL) | 1.7 (0.6-2.6) | 0.9 (0.5-1.9) | 0.029b | |
Table 2.
| Incidence proportion | Incidence ratea |
Before matching (n = 497) |
After matching (1:1) (n = 254) |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |||
| Catheterized | 9.30 | 2.90 | 12.48 (3.46-45.00) | < 0.001 | 13.15 (1.68-102.70) | 0.014 |
| Non-catheterized | 0.82 | 0.66 | Reference | - | Reference | - |
Table 3.
Two sensitivity analyses were conducted to investigate the association between catheterization and venous thromboembolism (VTE) development. In the multivariable logistic regression model, propensity scores (PSs) and catheterization were adjusted as covariates. In the other analysis, inverse probability of treatment weighted logistic regression model was used to estimate the OR for VTE development with the catheterization as the explanatory variable. OR, odds ratio; aOR, adjusted OR; CI, confidence interval.
Table 4.
| Variable | OR (95% CI) | P-value |
|---|---|---|
| Male sex | 0.94 (0.33-2.62) | 0.900 |
| Age (continuous) | 0.98 (0.95-1.02) | 0.344 |
| Body mass index | 0.86 (0.72-1.03) | 0.093 |
| Current smoking | 1.58 (0.49-5.08) | 0.443 |
| Disease | ||
| Ulcerative colitis | Reference | - |
| Crohn’s disease | 1.71 (0.61-4.81) | 0.307 |
| Disease duration (yr) | 0.96 (0.90-1.02) | 0.167 |
| Disease activity | ||
| Partial Mayo score | 1.09 (0.66-1.79) | 0.735 |
| Harvey-Bradshaw Index | 1.12 (0.93-1.34) | 0.235 |
| Past history of VTE | 4.85 (0.56-42.1) | 0.152 |
| Past history of surgery | 1.67 (0.58-4.79) | 0.337 |
| Treatment during admissiona | ||
| 5-ASA | 0.57 (0.20-1.60) | 0.284 |
| Azathioprine/6-MP | 0.55 (0.15-1.97) | 0.357 |
| Systemic corticosteroid | 1.39 (0.50-3.90) | 0.529 |
| Anti-TNF-α agent | 0.77 (0.21-2.78) | 0.691 |
| Vedolizumab | 1.20 (0.15-9.50) | 0.860 |
| Ustekinumab | 4.23 (0.50-36.18) | 0.188 |
| Tacrolimus | 0.31 (0.04-2.36) | 0.257 |
| Cyclosporin A | 3.38 (0.72-15.94) | 0.124 |
| Laboratory data on admission | ||
| White blood cells (/µL) | 1.00 (1.00-1.00) | 0.865 |
| Hemoglobin (g/dL) | 0.79 (0.64-0.97) | 0.027 |
| Platelet (×104/µL) | 1.00 (1.00-1.01) | 0.025 |
| C-reactive protein (mg/dL) | 1.08 (1.02-1.14) | 0.010 |
| Albumin (g/dL) | 0.28 (0.12-0.63) | 0.002 |
| D-dimer (µg/mL) | 0.93 (0.59-1.45) | 0.733 |









